Figure 1: General overview of glutathione synthesis and most important intracellular roles.
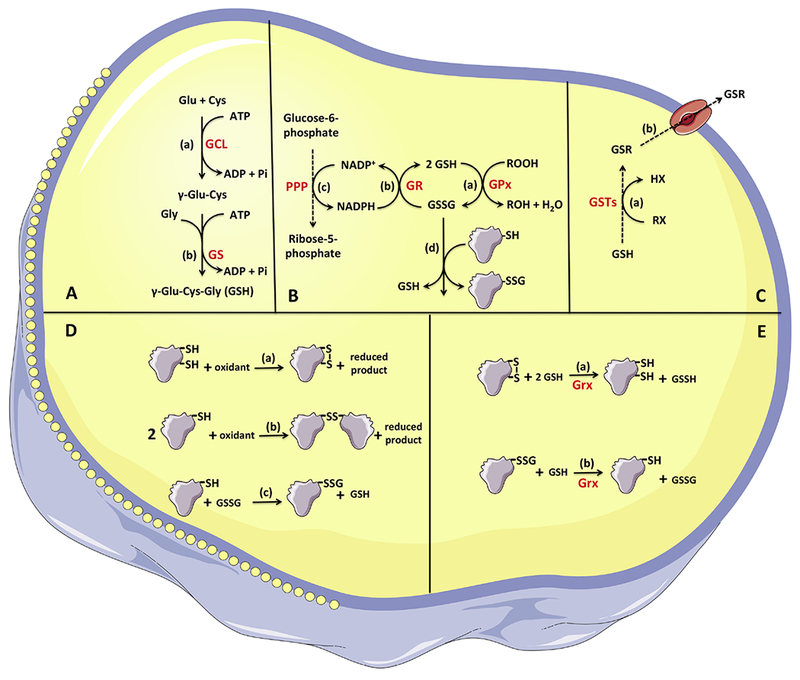
A) Glutathione synthesis - The tripeptide glutathione is synthesized in two ATP-consuming reactions. Event (a) represents the condensation of cysteine and glutamate to form the dipeptide γ-glutamylcysteine in a reaction catalyzed by glutamate cysteine ligase. Event (b) represents the addition of glycine to form the tripeptide in a reaction catalyzed by GSH synthetase. B) GSH-GSSG redox cycling - Glutathione peroxidases catalyze the reduction of H2O2 and organic peroxides at the expenses of electrons from GSH (event a). The oxidized glutathione generated by the GPx-catalyzed reaction can be reduced by glutathione reductase (event b), which uses NADPH generated mainly by the oxidative phase of the pentose phosphate pathway (event c). C) GSH complexation with xenobiotics - The conjugation of glutathione with diverse xenobiotics is catalyzed by glutathione-S-transferases (event a), forming a xenobiotic-GSH conjugate that can be exported from the intra- to the extracellular environment via multidrug resistance-associated proteins (event b). D) Thiol oxidation - The formation of protein disulfides can occur via either the oxidation of two protein thiols (from a single or different proteins, events a and b, respectively) or when a single sulfhydryl protein forms a disulfide with GSH via glutathionylation, forming a mixed disulfide (event c). E) Glutaredoxins - Glutaredoxins use electrons from glutathione to reduce oxidized target proteins, restoring the reduced state in cysteine(s) from proteins (event a) or from a mixed disulfide (event b).
GSH (reduced glutathione = γ-l-glutamyl-l-cysteinylglycine); GSSG (oxidized glutathione); Glu (glutamate); Cys (cysteine); γGluCys (dipeptide γ-glutamylcysteine); GCL (glutamate cysteine ligase); GS (GSH synthetase); GPx (glutathione peroxidase); GR (glutathione reductase); GSTs (glutethione-S-transferases); RX (xenobiotic); ROOH (peroxide = hydrogen or organic peroxide); Grx (glutaredoxin).
