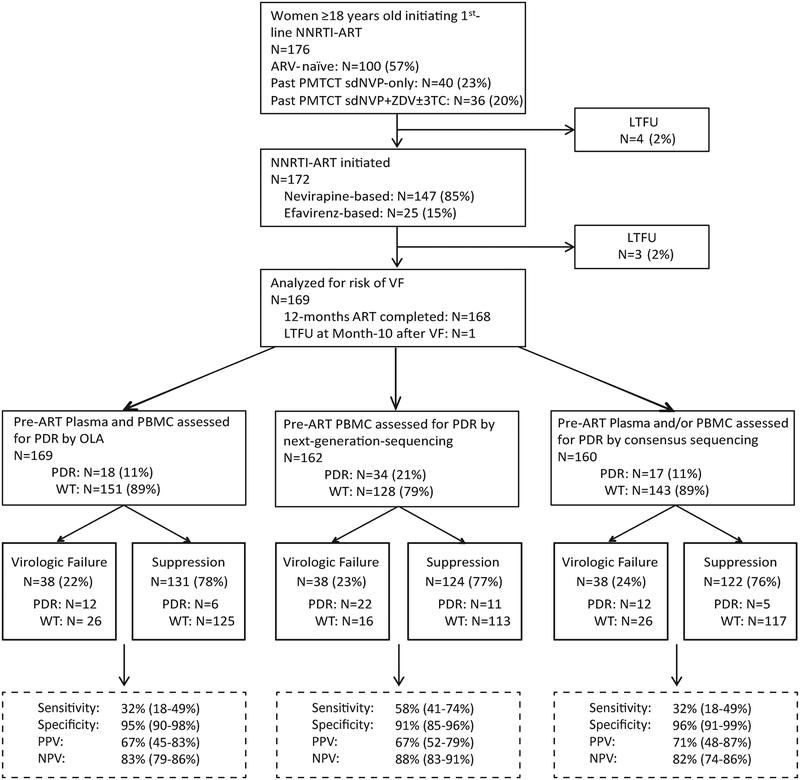
Figure 1. Schema of study of women initiating 1st-line non-nucleoside reverse transcriptase inhibitor (NNRTI)-based ART with retrospective assessments of pre-ART HIV drug-resistance (PDR), and virologic outcome at month-12 of study follow-up.
Enrolled participants either had no history of antiretroviral (ARV) treatment or a history of single-dose nevirapine (sdNVP) with or without prepartum zidovudine (ZDV) and/or post-partum ZDV ± lamivudine (3TC) for prevention-of-mother-to-child-transmission (PMTCT). Specimens were retrospectively assessed for PDR by an oligonucleotide ligation assay (OLA), consensus sequencing and next-generation-sequencing using the Illumina Miseq platform. Study outcomes, virologic failure (plasma viral load ≥400 HIV RNA copies/mL) or virologic suppression (plasma viral load <400 copies/mL) are shown, with contributions of participants with PDR vs. wild-type (WT) by each method of drug-resistance genotyping shown for each outcome. Diagnostic sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) have been calculated and presented for OLA, consensus sequencing, and next-generation-sequencing. Participants lost-to-follow-up (LTFU) are shown.