Abstract
Background:
Rotavirus (RV) can cause vomiting and diarrhea in infants and children, and could be treated clinically with immunoglobulin Y (IgY), which is an immunoglobulin extracted from chicken yolk. There is no systematic evaluation of immunoglobulin in the treatment of rotavirus enteritis. Therefore, we systematically evaluated rotavirus enteritis with oral immunoglobulin Y therapy using meta-analysis.
Methods:
We conducted a systematic search in CNKI, WANFANG DATA, VIP, PubMed, and the Cochrane Library databases (up to April 30, 2018). Using Revman 5.3 software for meta-analysis.
Results:
A total of 2626 subjects with rotavirus diarrhea from 17 randomized clinical trials were included in the meta-analysis. Of these, 1347 subjects received oral immunoglobulin Y and 1279 subjects received conventional treatment. The results of the meta-analysis indicated that the total number of effective cases and effective rates of immunoglobulin Y in treatment of rotavirus enteritis in infants and children was statistically different from that in the control group (odds ratio [OR] = 3.87, 95% confidence interval [CI] (3.17, 4.74), P < .00001) and (OR = 3.63, 95% CI [2.75, 4.80], P < .00001).
Conclusions:
Immunoglobulin Y is effective in the treatment of infantile rotavirus enteritis. Oral immunoglobulin Y can be widely used in the treatment of rotavirus enteritis in clinic.
Keywords: immunoglobulin Y, meta-analysis, rotavirus enteritis
1. Introduction
Rotavirus is an important pathogen causing acute diarrhea and death in infants. Nearly 600,000 infants die from rotavirus diarrhea every year.[1] Rotavirus diarrhea is a highly infectious and unpredictable disease. Rotavirus transmission through fecal and oral routes. The incubation period of rotavirus in vivo is 2 to 4 days, and the course of disease is generally about 2 weeks. At present, a large number of clinical evidence shows that the clinical symptoms of rotavirus infection are acute gastroenteritis with fever, abdominal pain, vomiting, diarrhea, and dehydration.[2]
Immunoglobulin Y (IgY) is a specific oral immunoglobulin extracted from the yolk of hens immunized with rotavirus and has the function of neutralizing, immunizing, and eliminating virus. In addition, IgY could change the surface configuration of the virus and prevent the virus from adsorbing to cells and bind to viruses, form immune complexes, and be engulfed by macrophages. Therefore, IgY is often used to treat diarrhea caused by rotavirus in clinic, however its efficacy has not been systematically evaluated by now. Herein, in this study, we investigated the clinical data of oral IgY in children with rotavirus enteritis using meta-analysis method, so as to provide evidence for the future clinical use of IgY in the treatment of rotavirus diarrhea.
2. Methods
Documents were retrieved from the database until April 2018. Specifically, we used the following search terms treated as Medical Subject Headings (MeSH) term or free text: “mianyiqiudanbai Y,” “baibeining,” “lunzhuangbingduchangyan,” “fuxie,” “Immunoglobulin Y,” “rotavirus,” “enteritis.” We conducted a systematic search in CNKI, WANFANG DATA, VIP, PubMed, and the Cochrane Library databases. A randomized controlled trial on oral immunoglobulin Y in infants with rotavirus enteritis was selected. Inclusion criteria include: referred to the 1998 “Diagnosis and Treatment of Diarrhea in China” or children diagnosed as rotavirus enteritis, their feces tested positive for antigen. The children were not limited to race, sex, and geography and excluded other complications. Exclusion criteria included: documents that did not obtain full text; documents that had duplicates, only 1 article; in vitro experiments; documents that were combined with other interventions. We observe the total number of effective cases (significant efficiency and efficient).
The methodological quality of the included studies was based on the Cochrane Risk Bias Assessment Tool. Data extraction: the 2 researchers took independent data collection through the included literature standards and extracted the title, first author, publication time, number of cases, number of valid cases, and number of total cases. If there is objection to the data, they will discuss the final determination.
The bivariate variables are expressed as an odds ratio (OR) and a 95% confidence interval (CI). Heterogeneity was assessed by using the Q statistic and I2 tests among trials. Heterogeneity was considered statistically significant when P < .1 or I2 > 40%. If heterogeneity existed, the data were analyzed using a random-effects model; if heterogeneity did not exist, a fixed-effects model was used.
All data analyses were performed by Revman software, version 5.3 (provided by Cochrane Collaboration). The final results were analyzed using the Forest plot and the publication bias was evaluated using the Funjnel plot.
This paper is a systematic analysis based on research data, so it does not need ethical approval.
3. Results
3.1. Search results
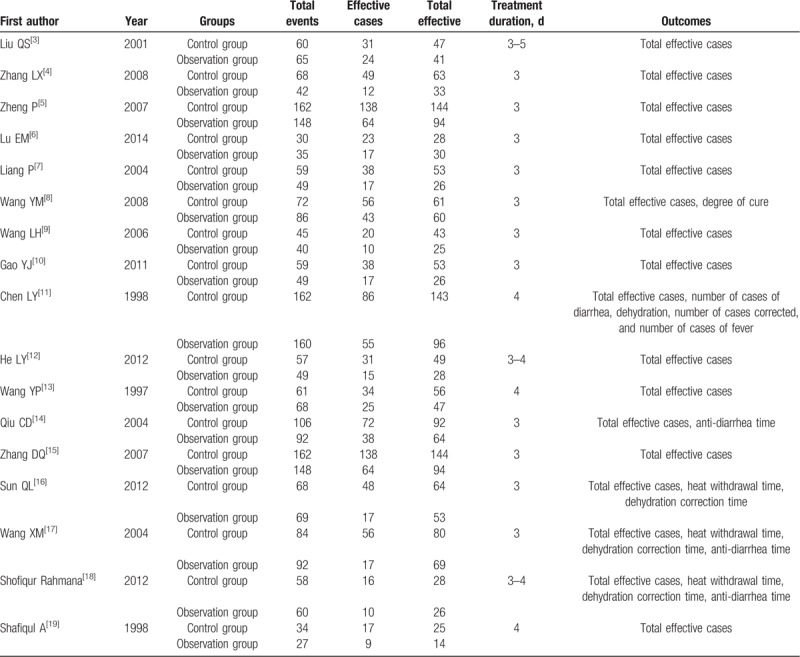
As shown in Fig. 1, 347 articles were retrieved. After reading the titles and abstracts, 300 duplicate research articles, meeting records, cell or animal research documents were deleted, and 47 articles were downloaded. Thirty of them were non-RTC studies, inconsistent drug use in experimental control group, combined with other drug treatment and evaluation indicators, which were excluded because of inconsistency with this paper. There are 15 Chinese articles[3–17] and 2 English articles.[18,19] A total of 2626 cases were treated with 1347 in the treatment group and 1279 in the control group. The basic situation of the literature is shown in Table 1.
Figure 1.
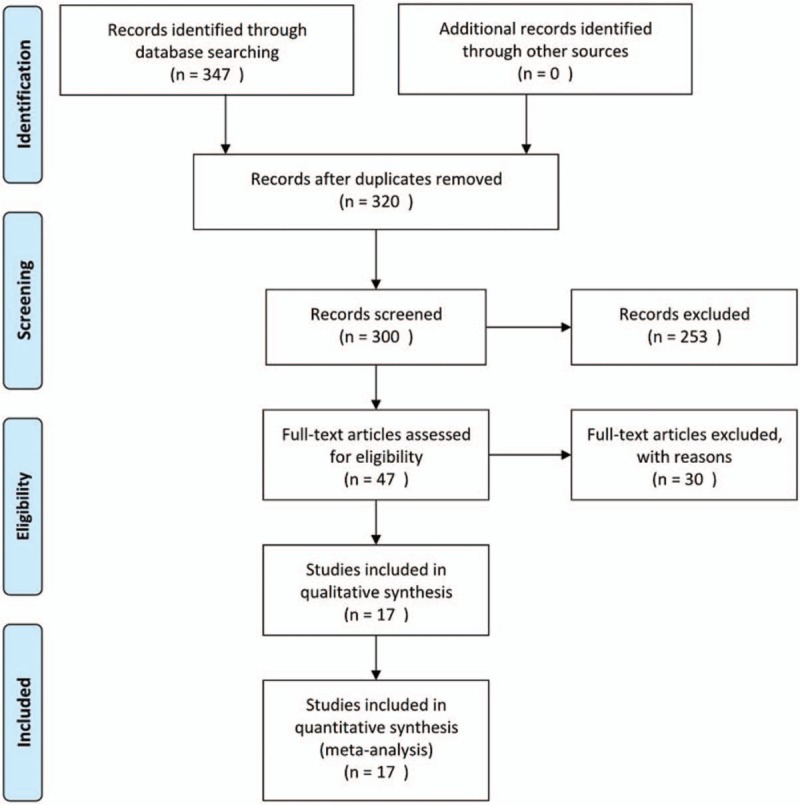
Screening process.
Table 1.
Characteristics of all included studies.
3.2. Meta-analysis results
3.2.1. Meta-analysis of total effective cases
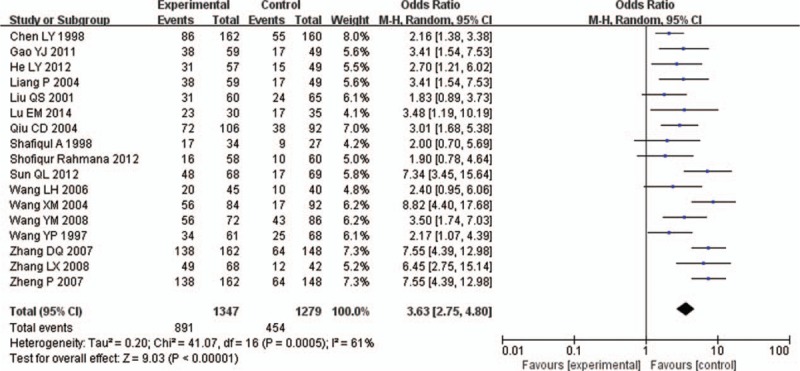
Chi-square test showed that there was slight heterogeneity between the 2 literatures (P = .09 I2 = 33%), Choosing Fixed Effect Model to analyze. Meta-analysis showed that the use of immunoglobulin Y increased the total effective cases in the treatment of rotavirus enteritis (OR = 3.87, 95% CI [3.17,4.74], P < .00001), as shown in Fig. 2.
Figure 2.
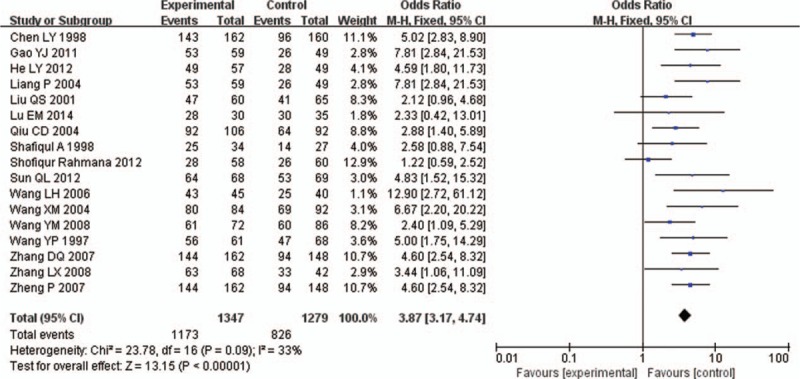
XXXXX.
3.2.2. Significant efficiency cases meta-analysis results
The literature reported the clinical significant efficiency rate after treatment, and the calculation method was: the number of significant effective cases/total cases. Chi-square test showed moderate heterogeneity among the literature (P = .0005, I2 = 61%), and random effects model was used for analysis. The results of meta-analysis showed that the use of immunoglobulin Y increased the number of significant efficiency cases in the treatment of rotavirus enteritis (OR = 3.63, 95% CI [2.75,4.80], P < .00001), as shown in Fig. 3.
Figure 3.
XXXXX.
3.2.3. Publishing bias analysis
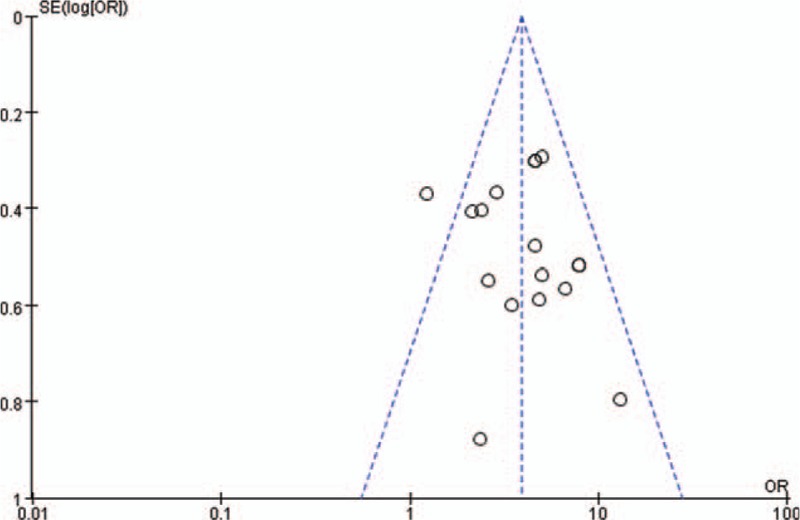
Using Revman 5.3 software to plot the total effective number of cases and the significant efficiency as the funnel plot of the outcome, which can be seen in the figure, each included document is represented by a dot. In the total number of effective cases forest plot, the points are symmetrical on both sides of the central axis, indicating that there is a light publication bias in each of the included documents as shown in Fig. 4; a funnel plot with significant efficiency as the outcome, with each point on both sides of the central axis. The distribution is relatively uneven, indicating that there is publication bias in each of the included documents, as shown in Fig. 5. The bias of each article is shown in Fig. 6.
Figure 4.
XXXXX.
Figure 5.
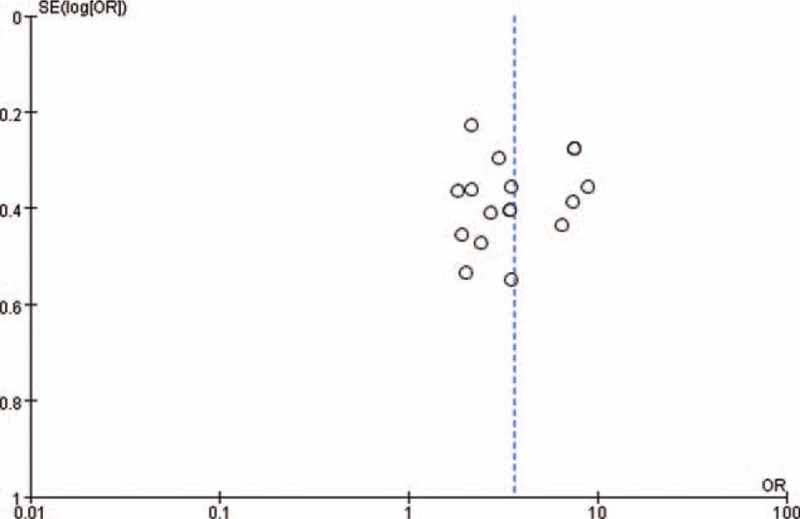
XXXXX.
Figure 6.
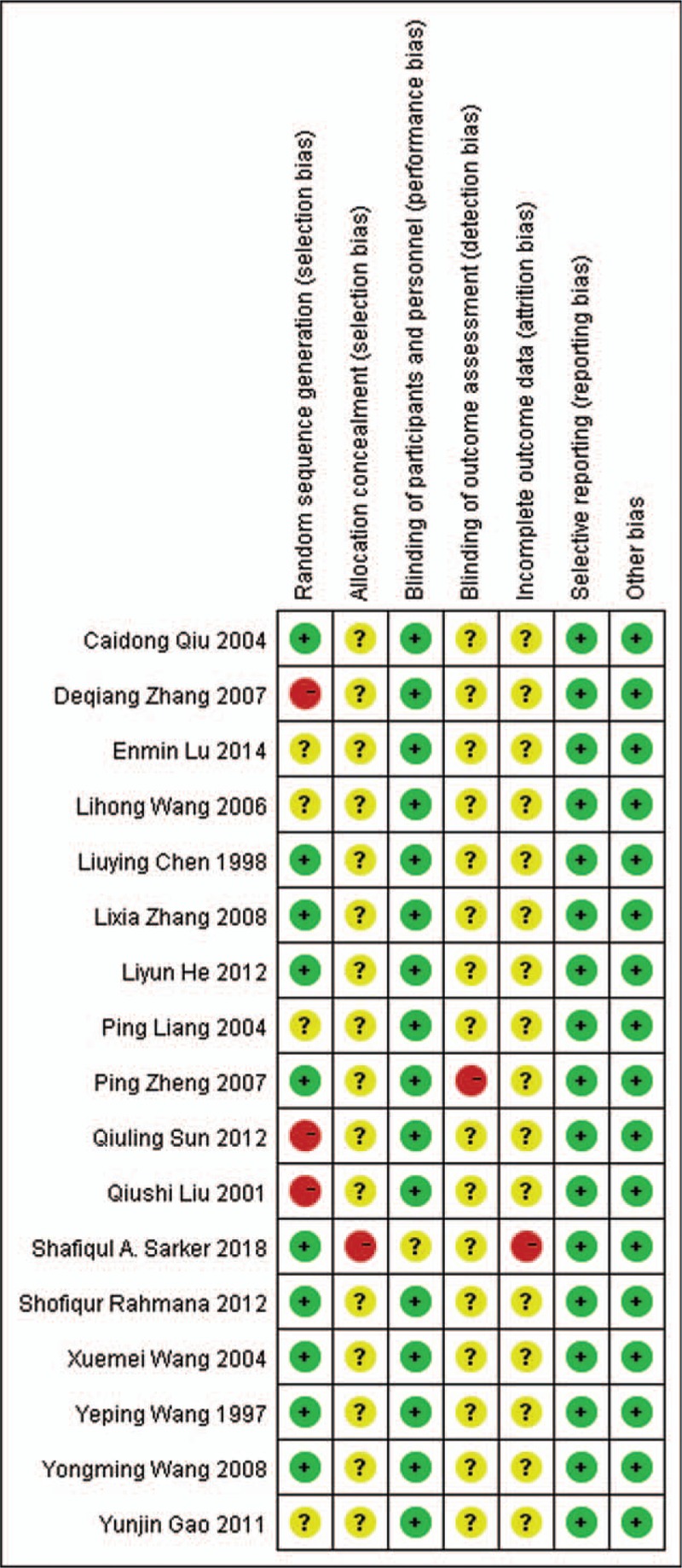
XXXXX.
3.2.4. Heterogeneity analysis
The causes of heterogeneity in the literature may be as follows: treatment time varies; the proportion of male and female children is inconsistent; the quality of the documents included in the survey was poor, and the score was low. The random production methods were not reported in detail, and bias may be generated.
4. Discussion
There is no effective drug for rotavirus infection at present. The most common and effective way to treat rotavirus diarrhea in clinic is to supply water and electrolytes. The drugs commonly used in the treatment of rotavirus diarrhea including: microecological agents. It can supplement microorganisms or promote the growth of beneficial microorganisms in the host and regulate the microecological balance. For example, Lasekan et al[20] added Lactobacillus Bb12 and TH4 in milk powder to inhibit the replication of the virus in vivo; antiviral drugs. These drugs mainly inhibit the synthesis of rotavirus nucleic acid and protein. For example, ribavirin is phosphorylated rapidly after into cells, resulting in a decrease in guanosine triphosphate, inhibiting the synthesis of viral RNA and protein, and inhibiting the replication and transmission of viruses.[21] Azim et al[22] found that the level of TNF-alpha in patients was lower than that before treatment, possibly because the immune response of the body was weakened and the synthesis of TNF-alpha was reduced. In addition, Solasodine, as an alkaloid compound, has anti-virus proliferation and anti-infection effects.[23] Ritterazine Y, as a newly synthesized marine drug, also has inhibitory effects on cells[24]; immunomodulator. Because the immune function of children is low, the use of immune regulators can enhance the immune function of the body artificially, thus treating rotavirus diarrhea. Thymosin can promote the maturation of T lymphocytes in peripheral blood, activate the secretion of various lymphokines, and increase the level of lymphokine receptors on T cells[25]; protective agent for gastrointestinal mucosa. The role of digestive tract mucosal protective agent is not only to protect the digestive tract mucosal barrier, but also to promote cell protection, mucus secretion, enhance mucus barrier, and promote mucosal repair. Gastrointestinal mucosal protective agent can increase the activity of T and B cells, increase IgA secretion, promote the recovery of intestinal mucosal function, improve the body's antiviral ability, and shorten the course of rotavirus enteritis.
IgY is a large number of RV antibodies produced in hens after RV was injected into them. Through mammalian immune transmission mechanism, antibodies are transferred to the embryo-egg system. IgY was obtained from immunized hen eggs by bioengineering and freeze-drying. This method makes IgY yield large, easy to purify, and good stability.[26] The mechanism of IgY in the treatment of rotavirus diarrhea may be as follows: inhibition of microbial adhesion to cell surface[27]; intercellular viral transmission was blocked; inhibition of enzyme activity; toxin activity neutralization.[28,29]
Oral IgY is a safe and effective choice for the treatment of neonatal rotavirus diarrhea. According to the research, it can effectively increase the total number of effective cases and remarkable curative effect, which confirms that it is superior to the traditional treatment of rotavirus enteritis. Therefore, oral immunoglobulin Y can be widely used in rotavirus diarrhea. At the same time, we infer whether infants can produce corresponding immunoglobulin Y to prevent rotavirus diarrhea by eating the eggs laid by immunized hens. For children with low birth weight or immunodeficiency, the efficacy and safety of IgY in controlling diarrhea caused by rotavirus infection are uncertain. Therefore, more randomized, placebo-controlled, and double-blind trials are needed.
Author contributions
Conceptualization: Xiaotong Wang.
Data curation: Xiaotong Wang, Wenpan Tan.
Formal analysis: Xiaotong Wang.
Funding acquisition: Lijun Song, Wenchang Zhao.
Investigation: Xiaotong Wang.
Methodology: Xiaotong Wang.
Project administration: Xiaotong Wang.
Resources: Xiaotong Wang.
Software: Xiaotong Wang.
Supervision: Lijun Song, Wenchang Zhao.
Validation: Xiaotong Wang.
Visualization: Xiaotong Wang.
Writing – original draft: Xiaotong Wang.
Writing – review & editing: Lijun Song, Wenpan Tan, Wenchang Zhao.
Wenchang Zhao orcid: 0000-0002-6195-0988.
Footnotes
Abbreviation: IgY = immunoglobulin Y.
XW and LS have contributed equally to this work.
The experiment was supposed by major science and technology projects of Guangdong province, China (2013A022100039); science innovation projects of higher school (2012KJCX0060); the technology bureau of Zhanjiang, Guangdong, China (2011C3108015); Guangdong province sail plan project of high level talents in 2014; the National Natural Science Foundation of China (81473401). Key project of Social science and technology development of Dongguan, NO. 20185071521658.
The authors report no declaration of interests.
References
- [1].Parashar UD, Hummelman EG, Bresee JS, et al. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis 2003;9:565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Barro M, Patton JT. Rotavirus NSP1 inhibits expression of type I interferon by antagonizing the function of interferon regulatory factors IRF3, IRF5, and IRF7. J Virol 2007;81:4473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Liu QS, Wu XL, Luo X, et al. Clinical observation of three methods in the treatment of rotavirus enteritis. Chin Phys J 2007;S1:85–6. [Google Scholar]
- [4].Zhang LX. Clinical analysis of Baibeining in the treatment of autumn diarrhea in children. Chin J Misdiagnos 2008;8:3555. [Google Scholar]
- [5].Zheng P, Sun XJ, Guo YH, et al. Therapeutic effect of anti-rotavirus immunoglobulin on infantile rotavirus enteritis. Chin Matern Child Health Care 2007;22:4108–9. [Google Scholar]
- [6].Liu EM. Anti-rotavirus immunoglobulin treatment of infantile rotavirus enteritis in 65 cases. Drugs People 2014;1:61. [Google Scholar]
- [7].Liang P. Treatment of infantile rotavirus enteritis with Baibeining. North China Pharma Sci 2004;01:35–6. [Google Scholar]
- [8].Wang YM. Baibening treatment of infantile rotavirus enteritis efficacy observation. China Pract Med 2008;3:92. [Google Scholar]
- [9].Wang LH. Analysis of the efficacy of baibeining in children with rotavirus enteritis in 45 cases. J Pract Med Technol 2006;13:3022. [Google Scholar]
- [10].Guo YJ. Treatment of infantile rotavirus enteritis with Baibeining. Anhui Med 2007;11:22. [Google Scholar]
- [11].Chen LY, Huang XQ, Hu YH, et al. Clinical application of anti-rotamy virus yolk immunoglobulin. Chin J Exp Clin Virol 1998;02:90–1. [Google Scholar]
- [12].He LY, Liu J. Clinical observation of rotavirus immunoglobulin treatment of rotavirus enteritis in infants and children. Yunnan Med 2012;03:242–4. [Google Scholar]
- [13].Wang YP, Wang HT, Liu QS, et al. Anti rotavirus chicken yolk immunoglobulin treatment of rotavirus enteritis efficacy observation. Chin J Exp Clin Virol 1997;4:81. [Google Scholar]
- [14].Qiu CD, Yao CJ, Fei HL, et al. Treatment of 106 cases of rotavirus enteritis with Baibeining. Chin J Metall Ind Med 2004;6:529–30. [Google Scholar]
- [15].Zhang DQ, Sun XJ. Baibeining in the treatment of rotavirus enteritis in children. Mod J Integrat Trad Chin West Med 2007;34:5122–3. [Google Scholar]
- [16].Sun QL. Treatment of pediatric rotavirus enteritis with Baibeining. Chin J Misdiagn 2012;12:2852. [Google Scholar]
- [17].Wang XM, Zheng XH, Zhang LY, et al. Treatment of pediatric rotavirus enteritis with Baibeining: a clinical analysis of 84 cases. Chin J Mod Clin Med 2004;9A:1382. [Google Scholar]
- [18].Shofiqur R, Kyoko HM, Khaing WH, et al. Randomized placebo-controlled clinical trial of immunoglobulin Y as adjunct to standard supportive therapy for rotavirus-associated diarrhea among pediatric patients. Vaccine 2012;30:466–9. [DOI] [PubMed] [Google Scholar]
- [19].Shafiqul A, Thomas H, Dilip M, et al. Successful treatment of rotavirus diarrhea in children with immunoglobulin from immunized bovine colostrum. Pediatr Infect Dis J 1998;17:1149–54. [DOI] [PubMed] [Google Scholar]
- [20].Lasekan JB, Jacobs J, Reisinger KS, et al. Lactose-free milk protein-based infant formula: imprat on growth and gastroin-testinal tolerance in infants. Clin Pediatr (Phila) 2011;50:330–7. [DOI] [PubMed] [Google Scholar]
- [21].Zhang J. Observation on the efficacy of ribavirin in the treatment of infantile rotavirus enteritis. J Clin Pract Hosp 2008;5:117. [Google Scholar]
- [22].Azim T, Ahmad M, Sefat-E-Khuda, et al. Immune response of children who develop persistent diarrhea following rotavirus infection. Clin Diagn Lab Immunol 1999;6:690–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kou Y, Koaq MC, Cheun Y, et al. Application of hypoiodite-mediated aminyl radical cyclization to synthesis of solasodine acetate. Steroids 2012;5:1069–74. [DOI] [PubMed] [Google Scholar]
- [24].Kou Y, Cheun Y, Koaq MC, et al. Synthesis of 14’,15’-dehydro-ritterazine Y via reductive and oxidative functionalizations of hecogenin acetate. Steroids 2012;2:304–11. [DOI] [PubMed] [Google Scholar]
- [25].Rasi G, Pierimarchi P, Sinibaldi Valllebona P, et al. Combination therapy in the treatment of chronic viral hepatitis and prevention of hepatocellular carcinoma. Int Immunopharmacol 2003;3:1169–76. [DOI] [PubMed] [Google Scholar]
- [26].Yokoyama H, Peralta RC, Sendo S, et al. Detection of passage and absorption of chicken egg yolk immunoglobulins in the gastro-intestinal tract of pigs by use of enzyme-linked immunosorbent as-say and fluorescent antibody testing. Am J Vet Res 1993;54:867–72. [PubMed] [Google Scholar]
- [27].Rahman S, Umeda K, Faustino C, et al. In vitro cytoprotective effect of infant milk formula fortified with human rotavirus-specific hyperimmune yolk immunogloblins (Ig Y). Food Sci Biotechnol 2013;22:1699–705. [Google Scholar]
- [28].Arimitsu H, Sasaki K, Kohda T, et al. Evaluation of Shiga toxin 2e-specific chicken egg yolk immunoglobulin: Production and neutralization activity. Microbiol Immunol 2014;58:643–8. [DOI] [PubMed] [Google Scholar]
- [29].Neri P, Tokoro S, Suqiyama T, et al. Recombinant Shiga toxin B subunit can induce neutralizing immunoglobulin Y antibody. Biol Pharm Bull 2012;35:917–23. [DOI] [PubMed] [Google Scholar]


