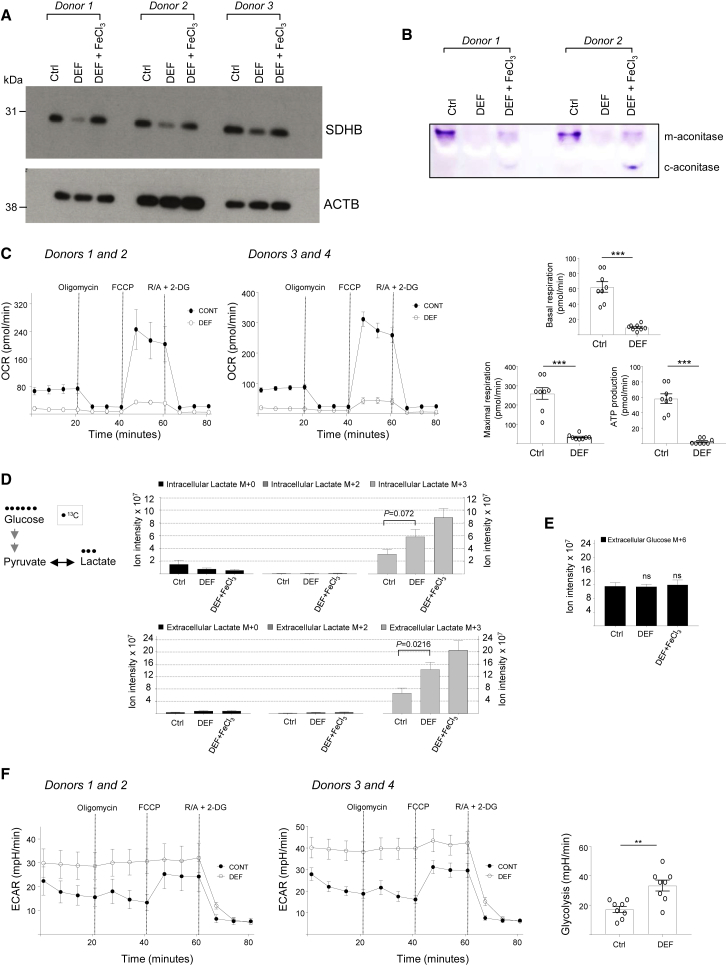
Figure 2.
Acute Iron Deprivation Abolishes Oxidative Phosphorylation and Increases Aerobic Glycolytic Flux in Human Macrophages
(A) SDHB western blotting in iron-deprived (DEF) and rescued (DEF + FeCl3) human macrophages from three donors. DEF (24 h, 500 μM); FeCl3 (8 h, 200 μM).
(B) Aconitase activity gel in iron-deprived (DEF) and rescued (DEF + FeCl3) human macrophages from two donors. m-aconitase, mitochondrial; c-aconitase, cytosolic.
(C) Oxygen consumption rate (OCR) measurement by extracellular flux analysis in control and DEF-treated human macrophages. Basal and maximal respiration and ATP production are shown at right. R/A, rotenone/antimycin; 2-DG, 2-deoxyglucose; DEF (24 h, 500 μM); n = 4 donors.
(D) Intracellular and extracellular lactate (glucose-derived adduct shown at left) isotopologue quantification by LC-MS in iron-deprived (DEF, 500 μM, 24 h) and rescued human macrophages with short exposure to 200 μM ferric chloride (DEF + FeCl3); n = 6 donors.
(E) Extracellular glucose M+6 by LC-MS; n = 6 donors.
(F) Extracellular acidification rate (ECAR) measured by extracellular flux analysis. R/A, rotenone/antimycin; 2-DG, 2-deoxyglucose, DEF (24 h, 500 μM); n = 4 donors.
Significance tested by t test (C, D, and F) and ANOVA followed by Dunnett’s multiple comparisons test (E). ns, non-significant compared to Ctrl. ∗∗p < 0.01 and ∗∗∗p < 0.001. Error bars represent SEM.