Abstract
Introduction:
Patients with dementia may be at a higher risk for death from stroke. We aimed to describe characteristics of dementia patients that died from ischemic stroke (IS) in Sweden.
Methods:
A retrospective longitudinal analysis of prospectively collected data of patients registered into the Swedish Dementia Registry was conducted. Data on causes of death, drugs and comorbidities were acquired from the Swedish nationwide health registers. Deaths were attributed to stroke if the death certificate contained stroke as a cause of death and the patient had a stroke registered in Riksstroke, the Swedish Stroke Register, in the year preceding death. Demographic data at the time of dementia diagnosis was compared between patients dying from IS and registered in Riksstroke, patients dying from IS without being registered in Riksstroke and those dying from other causes.
Results:
Out of 49823 patients diagnosed with dementia between 2007 and 2014 in primary care or specialist clinics, 14170 (28.4%) had died by the end of 2014. Of these 1180 (8.3%) had IS in their death certificate, of which 459 (38.9%) had been registered in Riksstroke. In patients who died of IS the most common type of dementia was vascular dementia while those died from other causes were most often diagnosed with Alzheimer’s dementia (AD). Patients who died from IS and were registered in Riksstroke had higher MMSE score compared to other groups. Patients who died from IS took more cardiovascular medications. There were no differences in the use of antipsychotics, antidepressants, acetylcholinesterase inhibitors, memantine, anxiolytics, or hypnotics between the groups.
Conclusions:
There was a relatively high number of patients who died from IS as shown in their death certificate but had not been registered in Riksstroke in the year before death. This creates concerns on the accuracy of death certificate stroke diagnoses, particularly for deaths taking place outside hospitals.
Keywords: Swedish dementia registry, SveDem, Swedish Stroke Registry, riksstroke, dementia, ischemic stroke, death certificate
1. INTRODUCTION
Mortality due to stroke has been declining over the past decades, most likely due to better management of vascular risk factors [1]. However, patients with dementia may be at a higher risk of dying from stroke [2]. Moreover, patients with dementia receive less intensive health care and they may not be appropriately managed for their cardiovascular comorbidities [3-5].
In the general population, stroke is the leading cause of death among older women and the second most frequent cause of death in Sweden overall [6]. In patients with dementia, the most common causes of death are bronchopneumonia and cardiovascular disease while death from neoplastic diseases is less common compared to the general older population [2, 7-10].
The severity of dementia may also influence the cause of death. While bronchopneumonia occurred more often in severely affected Alzheimer's disease (AD) patients, heart disease, stroke and other common causes of death predominated in less cognitively impaired patients [11]. In a recent study from SveDem stroke was a cause of death in 18.6% [10]. Dementia type is associated with cause of death - cerebrovascular diseases (CVD) are a significantly more common cause of death in vascular dementia [10, 12] patients compared to AD patients (33.3% vs. 9.3%) [10, 13]. However, most previous studies focused exclusively on AD patients and less information exists on stroke as a cause of death in other dementia types.
Death certificates are widely used in ascertainment of cause of death [14, 15]. Although their quality has been questioned, particularly in older individuals [15], a recent study with Swedish death certificates found an agreement of 87-88% for cardiovascular causes of death at the 3-digit level of the ICD-10 [16]. Swedish quality registers have a long tradition for improving quality of care and are often used in research. In a previous study from the Swedish Dementia Registry (SveDem), we examined the causes of death from death certificates according to dementia type and underlying demographic factors [10]. Now, we propose a study focusing on ischemic stroke (IS), with the aim to describe the characteristics of patients with dementia dying from stroke and to determine what proportion of patients with a stroke in their death certificate had been diagnosed with stroke before death. A cross-reference with the Swedish Stroke Register [17] was performed to determine whether individuals received a stroke diagnosis before the terminal event, in an effort to increase the reliability of the death certificate diagnoses. Lastly, we aimed to establish whether the place of death contributed to missing clinical stroke diagnoses in patients dying from stroke according to their death certificates.
2. MATERIALS AND METHODS
A retrospective analysis of prospectively collected data of patients registered in the quality Swedish Dementia Registry (SveDem) was conducted. Information about the causes of death was acquired from the Swedish Death Register, about comorbidities from the Swedish Patient Register and about drugs from the Swedish Prescribed Drugs Register. Patients and their relatives were informed of the entry into SveDem and could decline participation or have their data removed at any time. Data were de-identified before analysis. This study complies with the Declaration of Helsinki and was approved by the regional ethical review board in Stockholm, Sweden (dnr 2015/743-31/4).
2.1. Source of Data
The Swedish Dementia Registry (SveDem) was established in May 2007, with the aim to monitor the care of patients with dementia in Sweden, as previously described in detail [18]. Patients with dementia are registered at the time of their dementia diagnosis by physicians in memory clinics or primary care units. Diagnoses were made according to the International Classification of Diseases version 10 (ICD-10) criteria, [19] with specific criteria used for the different dementia types. The following dementia disorders are included in SveDem and were considered for this study: Alzheimer's dementia (AD), vascular dementia [12], mixed AD and VD (mixed), dementia with Lewy bodies (DLB), frontotemporal dementia (FTD), Parkinson's disease dementia (PDD) and unspecified dementia (UD). Information on age, sex, Mini-Mental State Examination (MMSE) score, use of medication and living conditions are recorded. In the present study, we use data of patients that were registered between 2007 and 2014.
Data on death were obtained from death certificates collected in the Swedish Death Register until December 2014. IS was included as the cause of death if it was mentioned either as the main or contributory cause (ICD-10 code I63). All other causes of death were included in the variable “dead from other causes”. The Swedish Stroke Registry covers all Swedish hospitals involved in acute stroke with a coverage >90% for IS events [17] and was used to ascertain stroke diagnoses [20]. Stroke events occurring between 2007 and 2014 were included. Deaths were attributed to stroke if the death certificate contained stroke as the cause of death and the patient had a stroke registered in Riksstroke within 1 year prior to death. The place of death was either hospital, nursing home or at home.
Information on comorbidities was obtained from the Swedish Patient Registry that covers all hospital and specialist care in Sweden [21]. One main diagnosis and up to 21 additional diagnoses are registered with ICD 10 codes. In the present study, we considered a comorbidity present if the code appeared at least once as the main or additional diagnosis from 1998 until the end of 2014. We used the following codes for comorbidities: diabetes mellitus (E10-E13), arterial hypertension (I10-I15), anemia (D50 and D62), liver diseases (K70-77), kidney diseases (N10-19), heart failure (I50), atrial fibrillation (I48), previous femur fracture (S72), previous stroke (I64, I63, I60-I62).
Information on drug use was obtained from the Prescribed Drugs Registry which contains information on all dispensed prescriptions since July 2005 at Swedish pharmacies to the entire Swedish population [22]. Drugs are coded according to the Anatomical Therapeutic Chemical (ATC) Classification system. In the present study, we analyzed drugs dispensed at the time of dementia diagnoses and up to 3 years prior to this date, using the following codes: antidiabetic drugs (A10), antithrombotic agents (B01), diuretics (C03), beta blocking agents (C07), calcium-channel blockers (C08), agents acting on the renin-angiotensin system (C09), lipid-modifying agents (C10), antipsychotics (N05A), antidepressants (N06A), cholinesterase inhibitors (ChEIs) (N06DA), memantine (N06DX01), anxiolytics (N05B), hypnotics and sedatives (N05C). The use of sleeping aids was obtained from SveDem since ATC codes do not always reveal the intention of the prescription. Further, we consider the total number of medication at the time of dementia diagnosis as a proxy for overall co-morbidity.
2.2. Statistical Analyses
Data is described as means ± standard deviation (SD), median and interquartile range (IQR) or frequency (number-n and percentage-%), where appropriate. Baseline characteristics of the patients at the time of dementia diagnosis were compared between patients dying from IS and registered in Riksstroke, patients dying from IS without being registered in Riksstroke and those dying from other causes using chi-square test or T-test, as appropriate. Age was compared using ANOVA with the Welch test because of non-homogenous variances and the total number of drugs using the Kruskal-Wallis test. Logistic regression was used to assess the association of place of death (hospital, nursing home or at home) with IS in the death certificate and having a Riksstroke registration. The final models were adjusted for age, sex, number of medication, MMSE, dementia type, previous diagnosis of diabetes, hypertension, heart failure, atrial fibrillation, stroke, anemia, hip fracture, liver diseases and kidney diseases. Adjusted odds ratios (ORs) with 95% confidence intervals (CIs) are presented.
Two-tailed P < 0.05 was considered to be statistically significant in all analytical procedures. Data were analysed using the Statistical Package for the Social Sciences software version 22 (IBM Corporation, Armonk, NY, USA).
3. RESULTS
Out of 49823 patients diagnosed with dementia between 2007 and 2014 in primary care or specialist clinics, 14170 (28.4%) had died by the end of 2014. Of these, 1180 (8.3%) had IS mentioned in their death certificate, of which 459 (38.9%) had also been registered in Riksstroke for a stroke event in the year preceding death. Characteristics of the cohort are presented in Table 1. Of patients who died of IS, there were additionally 153 patients who had a Riksstroke registration more than a year preceding death, totaling 612 patients with a confirmed IS or 52% of those with IS mentioned on their death certificates. Characteristics of this cohort are presented in Supplemental Table 1 (363KB, doc) .
Table 1. Baseline characteristics of patients.
| - |
Died from Ischemic Stroke and Registered in
Riksstroke1 (N=459) |
Died from Ischemic Stroke, not in Riksstroke (N=721) | Death from Other Causes (N=12990) | P Value |
|---|---|---|---|---|
| Place of death | ||||
| Hospital | 257 (56.0%) | 121 (16.8%) | 3266 (25.1%) | <0.001† |
| Nursing home | 182 (39.7%) | 522 (72.4%) | 8009 (61.7%) | |
| At Home | 6 (1.3%) | 30 (4.2%) | 705 (5.4%) | |
| Unknown/blank | 4 (3.1%) | 48 (6.7%) | 1010 (7.8%) | |
| Age at diagnosis, mean ± SD | 82.5 ± 6.1 | 82.3± 6.8 | 81.9 ± 7.2 | 0.042‡ |
| Age at death, mean ± SD | 84.5 ± 6.0 | 84.6 ± 6.6 | 84.3 ± 7.0 | 0.434‡ |
| Female gender, n (%) | 274 (59.7%) | 404 (56.0%) | 7138 (54.9%) | <0.118† |
| Dementia type, n (%) | ||||
| AD | 90 (19.6%) | 120 (16.6%) | 3298 (25.4%) | <0.001† |
| Mixed | 106 (23.1%) | 161 (22.3%) | 2661 (20.5%) | 0.209† |
| Vascular | 136 (29.6%) | 227 (31.5%) | 2684(20.7%) | <0.001† |
| DLB | 7 (1.5%) | 17 (2.4%) | 362 (2.8%) | 0.122† |
| FTD | 3 (0.7%) | 5 (0.7%) | 188 (1.4%) | 0.096† |
| PDD | 4 (0.9%) | 9 (1.2%) | 249 (1.9%) | 0.124† |
| Unspecified | 106 (23.1%) | 166 (23.0%) | 3257 (25.1%) | 0.306† |
| Other | 7 (1.5%) | 16 (2.2%) | 289 (2.2%) | 0.604† |
| MMSE, median (IQR) | 21.0 (7) | 20.0 (9) | 20.0 (9) | <0.001 |
| 0 to 17 | 155 (33.8%) | 305 (42.3%) | 5133 (39.5%) | 0.013† |
| 18 to 22 | 129 (28.1%) | 238 (33.0%) | 3962 (30.5%) | 0.860† |
| 23 and over | 175 (38.1%) | 178 (24.7%) | 3895 (30.0%) | <0.001† |
| Total number of drugs, median (IQR) | 6.0 (5) | 6.0 (6) | 5.0 (5) | <0.001§ |
| Antidiabetics (A10) | 62 (13.5%) | 107 (14.8%) | 1454 (11.2%) | 0.004† |
| Antithrombotic agents (B01) | 270 (58.8%) | 412 (57.1%) | 5949 (45.8%) | <0.001† |
| Diuretics (C03) | 158 (34.4%) | 233 (32.3%) | 3859 (29.7%) | 0.036† |
| Beta blocking agents (C07) | 212 (46.2%) | 239 (33.1%) | 4227 (32.5%) | <0.001† |
| Calcium-channel blockers (C08) | 79 (17.2%) | 127 (17.6%) | 1981 (15.3%) | 0.130† |
| Agents acting on the renin-angiotensin system (C09) | 165 (35.9%) | 231 (32.0%) | 3476 (26.8%) | <0.001† |
| Lipid-modifying agents (C10) | 116 (25.3%) | 189 (26.2%) | 2656 (20.4%) | <0.001† |
| Antipsychotics (N05A) | 30 (6.5%) | 63 (8.7%) | 1050 (8.1%) | 0.388† |
| Antidepressants (N06A) | 108 (27.1%) | 214 (29.7%) | 3514 (27.1%) | 0.067† |
| Cholinesterase inhibitors (N06DA) | 157 (35.3%) | 222 (32.8%) | 4632 (37.2%) | 0.053† |
| Memantine (N06DX01) | 43 (9.7%) | 65 (9.7%) | 1300 (10.5%) | 0.665† |
| - |
Died from Ischemic Stroke and Registered in
Riksstroke1 (N=459) |
Died from Ischemic Stroke, not in Riksstroke (N=721) | Death from Other Causes (N=12990) | P Value |
| Cholinesterase inhibitors in patients with AD or mixed dementia (N06DA) | 107 (56.0%) | 147 (53.8%) | 3281 (56.0%) | 0.777† |
| Anxiolytics (N05B) | 55 (12.6%) | 115 (17.3%) | 1785 (14.7%) | 0.081† |
| Sleeping aids (N05C) | 98 (22.5%) | 151 (22.48%) | 2497(20.6%) | 0.249† |
1Died from ischemic stroke and registered in Riksstroke within the past year. N, number of patients in each category; SD, standard deviation; IQR, interquartile range; MMSE, Mini Mental State Examination score. AD, Alzheimer's dementia; mixed, mixed Alzheimer's and Vascular dementia; DLB, Dementia with Lewy Bodies; FTD, Frontotemporal dementia; PDD, Parkinson's disease with dementia. P-values obtained from †Chi-Square, ‡ANOVA with Welch test because of non-homogenous variances, § Kruskal-Wallis.
Among patients who died of IS, the most common type of dementia was VD, while those dead from other causes were most often diagnosed with AD. Patients who died from IS and were registered in Riksstroke had higher MMSE score compared to other groups (21.0 vs. 20.0 vs. 20.0; p<0.001).
56.0% of patients died from IS and registered in Riksstroke died in hospital compared to 16.8% patients who died from IS without being registered in Riksstroke, and 25.1% who died from other causes. Patients who died from IS were slightly older when registered in SveDem, but in all groups patients died with a mean age of 84 years.
Patients who died from IS took more medications at the time of dementia diagnosis, when compared to patients who died from other causes (6.0 vs. 5.0; p<0.001). Specifically, they were prescribed more cardiovascular drugs, such as antithrombotic agents, beta-blockers, agents acting on the renin-angiotensin system, lipid-modifying agents and antidiabetics. There were no statistically significant differences in the use of antipsychotics, antidepressants, ChEIs, memantine, anxiolytics, or hypnotics between the groups. Fig. (1) shows medication use at the time of dementia diagnosis in patients who died from IS and those who died from other causes.
Fig. (1).
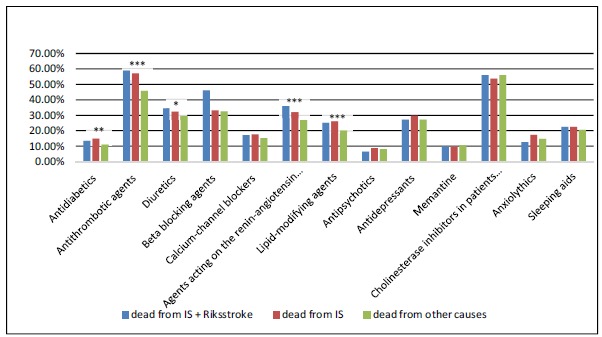
Medication use at the time of dementia diagnosis.
Dying in a hospital was associated with increased odds of having a stroke in the death certificate and being registered in Riksstroke (Table 3). Compared to patients dying in a hospital, those dying in a nursing home had lower odds of having IS in their death certificates (OR 0.80, 95% CI 0.69-0.92) and, among those dying from IS, nursing home residents were less likely to present a Riksstroke registration within one year of death (OR 0.17, 95% CI 0.12-0.22).
Table 3. Odds ratios (OR) of having ischemic stroke (IS) and a Riksstroke registration in the death certificate, relative to place of death.
| - | OR for having IS (95% CI) | OR for being Registered in Riksstroke (95% CI) |
|---|---|---|
| Hospital | Ref. | Ref. |
| Nursing home | 0.80 (0.69-0.92)*** | 0.17 (0.12-0.22)*** |
| At Home | 0.49 (0.34-0.70)*** | 0.09 (0.04-0.23)*** |
Logistic regression was used to assess the association of place of death (hospital, nursing home or at home) with IS in the death certificate and of having a Riksstroke registration within the year prior to death. Adjusted for age, sex, number of medication, Mini-Mental State Examination (MMSE), dementia type, previous diagnosis of diabetes, hypertension, heart failure, atrial fibrillation, stroke, anemia, hip fracture, liver diseases and kidney diseases. Adjusted odds ratios (ORs) with 95% confidence intervals (CIs) are presented. * P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001.
4. DISCUSSION
There were 8% of patients with dementia in our study who died from IS, which is slightly higher than the 6% that die from IS in the general population [23]. Some studies evaluated stroke as a cause of death in dementia patients [2, 7, 8, 11, 13] providing conflicting results. Some showed that death because of CVD was more common in dementia patients than in the general population [2] while others did not demonstrate a difference, [8] or showed the opposite results [7]. Death from CVD accounted for around 4-13% of all deaths in patients with dementia in different studies [2, 7].
In our cohort, only one-third of all patients who died of IS had been registered in Riksstroke for a stroke event in the year preceding death. There are a few possible explanations for this low number of registrations in Riksstroke. First, it is estimated that around 10% of strokes may be missed by Riksstroke (90% coverage) [17]. Further, some patients with strokes may survive more than one year from the stroke event and would have been excluded from this study: in these cases, however, it may be hard to attribute the death to stroke. Therefore we performed a secondary analysis including all patients with previous Riksstroke registration irrespective of the time between stroke and death. This increased the percentage of patients with an established stroke diagnosis to 52% but did not change the findings of previous analysis (Supplemental Table 1 (363KB, doc) , 2 (363KB, doc) , 3 (363KB, doc) ). In a previous study examining mortality after IS, the highest mortality rate occurred in the first 3 months after IS but remained elevated even among patients who survived 2 years [24] . In the first month after IS, deaths were caused primarily by neurologic complications of brain ischemia, while later deaths were mostly associated with cardiac and pulmonary events [24]. Similarly, another study examining long-term survival after stroke found that stroke was associated with an almost 5-fold increase in risk for death between one month and one year after the first stroke and a 2-fold increase after 1 year compared with the general population. In this study, almost half of the patients died one year after the stroke [25]. In another study that examined early and long-term causes of death after IS, stroke caused early deaths in 57% and only 14% long-term deaths [26].
Another important point is the accuracy of death certificate diagnosis. Different definitions of stroke as a cause of death in literature make comparisons between studies challenging. Beside this, it is well-known among clinicians that the diagnosis of stroke in a patient with severe dementia is complicated due to impaired communication and cooperation at examination [27]. However, generally high error rates appeared in death certification - approximately 33-41% of
Antidiabetics (A10), antithrombotic agents (B01), diuretics (C03), beta blocking agents (C07), calcium-channel blockers (C08), agents acting on the renin-angiotensin system (C09), lipid-modifying agents (C10), antipsychotics (N05A), antidepressants (N06A), cholinesterase inhibitors (N06DA), memantine (N06DX01), anxiolytics (N05B), sleeping aids (N05C). P values obtained from Chi-Square tests. * P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001.
incorrectly identified causes of death, with disproportionate overrepresentation of cardiovascular events [28]. For example, data from the Framingham Heart Study suggests that cardiovascular causes of death are overstated in at least 24% of cases [29]. There was also concern over the overestimation of stroke diagnosis as the cause of death when there is a history of stroke and a lack of an obvious specific cause of death [25]. In data from the Framingham Study, a high error rate was shown for stroke, including a 40% false negative rate in death certificates, which significantly increased with increasing age at death and increasing interval from the last stroke to death [30]. If death occurred within one month, the frequency of false negatives was 7%, but for those who survived more than one month, it became 59%. Another Swedish study that examined stroke incidence and survival raised concern about underestimation of stroke incidence in previous retrospective studies [31]. They prospectively screened for patients with stroke, using multiple overlapping case ascertainment methods and emphasized the importance of using recommended criteria for an ideal epidemiological stroke study [32]. However, a previous study in Sweden found that the main diagnosis from the patient's final hospital discharge record differed from the original underlying cause of death in the death certificate in more than half of all cases [33]. This contradicts other reports on good accuracy of Swedish death certificates [16].
Having stroke in the death certificate and being registered in Riksstroke was associated mostly with the place of death - dying in a hospital was associated with increased odds compared to dying at home or in a nursing home. This can be explained because Riksstroke is by definition a hospital based register, so patients who have a stroke at nursing homes or at home and who are not admitted to hospital will not be included in the register. Stroke is a clinical diagnosis but in order to characterize an event as ischemic or hemorrhagic a CT scan of the brain is needed and this can only be performed in hospitals, making stroke primarily a hospital diagnosis. The current stroke guidelines from the Swedish Board of Health and Welfare accept that some very frail patients with comorbidities or end-of-life stages may not be transferred to the hospital when they suffer a stroke [34]. They recommend that this decision be taken in anticipation of a potential future stroke event, individualized to a particular patient and after discussion with the patient and care providers. However, when patients from nursing homes have received hospital care they are registered in Riksstroke, even if the final place of death is a nursing home. In a recent Swedish study, hospitalization proportions for patients with acute stroke were around 10% lower than previous estimations. 84% of patients were hospitalized with a median duration of 7 days, 8% were examined at a hospital but not hospitalized, 4% were treated within the primary care organization, 1% died before medical examination and 3% had no contact with medical services [35]. Another study analysing late follow-up after stroke (three months to one year), found that 46% of patients died in a nursing home, whereas 37% of patients died in a hospital and 10% of patients died at home, which is a similar distribution compared to our study [36].
4.1. Type of Dementia in Patients who Died from Stroke
As expected from previous work with causes of death in SveDem, we found that most common type of dementia in patients who died from stroke was VD [10]. VD is by definition associated with CVD and therefore patients with VD also have higher prevalence of cardiovascular risk factors [36].
The most common type of dementia in patients who died from other causes was AD. Some studies showed that patients with dementia have an increased risk of stroke [37], others showed that patients with AD did not have an altered risk of ischemic stroke compared to those without dementia, whereas patients with VD had an approximately 2-fold increased risk [38].
Less is known about stroke in other dementia types. In PD, previous studies have shown conflicting results on the relationship between IS and PD. To the best of our knowledge there is little data on stroke mortality in PDD, but stroke-related mortality was estimated to be 1.5 times higher in patients with PD compared to the general population [39]. However, in two case-control studies, the risk of stroke was lower or not different in PD [40, 41]. In a study on mortality in PD, 8% of deaths were from cerebrovascular disease [42].
There are also few and conflicting results regarding the relationship between IS and DLB, with studies reporting both increased [43] and decreased risk [44].
There was a lower prevalence of cerebrovascular diseases in FTD compared to patients with non-FTD dementia (12.7% vs. 26.1%) but these differences became non-significant after adjusting for age [45].
To conclude, the data on stroke mortality in different types of dementia is still scarce and more research is needed.
4.2. Medications in Patients Dead From Stroke
In our study, we found that patients who died from stroke did not differ in the use of antipsychotics, antidepressants, ChEIs, memantine, anxiolytics or hypnotics compared to patients who died from other causes. This is contrary to previous studies showing that patients who were taking anxiolytic and hypnotic drugs presented with significantly increased risk of mortality and higher prevalence of stroke (10% vs.7%; P ≤ 0.001) [46]. In recent years, concern has been raised about the increased risk of stroke with the use of antipsychotics, both in general population and patients with dementia [47, 48]. The risk of death was about 1.2 to 1.6 times higher compared to the no-treatment group in some studies [49, 50]. Because of higher awareness of possible side effects, the use of antipsychotics has been restricted in patients with dementia. A nationwide study from Denmark observed a decrease in the prevalence of antipsychotic use among patients with dementia from 31.3% in 2000 to 20.4% in 2012 [51]. This was accompanied by a decrease in anxiolytics and hypnotics/sedatives, but with an increase in the use of antidepressants from 43.3% in 2000 to 53.8% in 2012 [51]. In comparison, patients in our study used antipsychotics at a lower rate - 6.5% in patients with dementia who died from stroke and registered in Riksstroke compared to 8.1% who died from other causes [52].
4.3. Limitations and Strengths
This study is strengthened by a large sample of patients from different health care setting, reflecting the current clinical practice in Sweden, and the utilization of several nationwide registers. The information on causes of death, drugs and comorbidities was derived from nationwide health registers that have a complete coverage in the country. Furthermore, Riksstroke has an excellent coverage in Sweden, containing > 90% of stroke events in the country [53]. Some limitations need to be mentioned. In the period examined, 52% of memory clinics and 48% of primary care units in Sweden were affiliated to SveDem and patients in SveDem may differ from non-registered patients. It was suggested that patients included in a quality register are more likely to be men, younger, generally healthier and of a higher socioeconomic status [4]. If this holds for SveDem, it may limit the generalizability of our findings to a healthier and younger population that has more contact with health care, possibly leading to an underestimation of the rate of deaths from IS.
Another weakness of this study is the absence of cognitively normal controls. The ascertainment of stroke as the cause of death is obviously a well-founded concern, particularly considering the discrepancies found between stroke as the cause of death and stroke registered in Riksstroke.
This is an observational study, so it is impossible to establish causation between different medications and outcomes.
The validity of the data in SveDem is subject to random cross-checks of histories and entries [18] and less than 5% of dementia diagnoses change during the first year of follow-up [54].
CONCLUSION
Mortality data is a cornerstone of epidemiological research and health monitoring. At both the state and national level, mortality data compiled from death certificates is used to track disease trends, set public health policies, and allocate health and research funding [55]. Quality issues may impact health care policy. The discrepancy between the registration of IS in death certificates and in Riksstroke is a cause for concern. In future, studies on non-dementia patients registered in Riksstroke could determine whether this is a widespread issue or particular of patients with dementia.
Table 2. Comorbidities at dementia diagnosis.
| - | Died from Ischemic Stroke and Registered in Riksstroke (N=459) |
Died from Ischemic Stroke, not in
Riksstroke (n=721) |
Death from Other Causes (N=12990) | P Value |
|---|---|---|---|---|
| Comorbidities at dementia diagnosis | ||||
| Diabetes mellitus, n (%) | 88 (19.2) | 140 (19.4) | 2046 (15.8) | 0.006 |
| Arterial hypertension, n (%) | 265 (57.7) | 371 (51.5) | 5705 (43.9) | <0.001 |
| Anemia | 34 (7.4) | 38 (5.3) | 839 (6.5) | 0.308 |
| Liver disease, n (%) | 2 (0.3) | 4 (0.9) | 147 (1.1) | 0.088 |
| Kidney disease, n (%) | 43 (9.4) | 49 (6.8) | 1074 (8.3) | 0.250 |
| Heart failure, n (%) | 102 (17.4) | 122(16.9) | 2264 (17.4) | 0.027 |
| Atrial fibrillation, n (%) | 191 (41.6) | 193 (26.8) | 2859 (22.0) | <0.001 |
| Previous femur fracture, n (%) | 50 (10.9) | 90 (12.5) | 1435 (11.0) | 0.485 |
| Previous stroke, any type n (%) | 155 (33.8) | 291 (40.4) | 2437 (18.8) | <0.001 |
1Died from ischemic stroke and registered in Riksstroke within the past year. Any type stroke refers to both ischemic and hemorrhagic. P values obtained from Chi-Square tests.
ACKNOWLEDGEMENTS
The authors are grateful to SveDem and Riksstroke and all patients, caregivers and staff. SveDem is supported by the Swedish Association of Local Authorities and Regions and Swedish Brain Power. This study has been financially sup- ported by FORTE, the Swedish Research Council for Health, Working Life and Welfare (dnr 2017-01646), Johanniteror- den i Sverige/Swedish Order of St John, the Swedish Stroke Association, Loo and Hans Osterman's Foundation for Medi- cal Research, the Foundation for Geriatric Diseases at Karolinska Institutet, the Foundation to the Memory of Sigurd and Elsa Goljes, Gun and Bertil Stohne's Foundation, Margaretha af Ugglas’ Foundation, Swedish Research Coun- cil (523-2012-2291), Stiftelsen Dementia and Sustainability for the National Institute of Mental Health (grant LO1611), with a financial support from the Ministry of Education, Youth and Sports of the Czech Republic. The funding orga- nizations did not participate in study design or data interpre- tation.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the regional ethical review board in Stockholm, Sweden (dnr 2015/743-31/4).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All humans research procedures were accordance with the Declaration of Helsinki.
CONSENT FOR PUBLICATION
Patients and relatives were informed of inclusion in the registries at the time of diagnosis and could decline participation or withdraw consent. Data were de-identified before analysis.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s web site along with the published article.
REFERENCES
- 1.Lackland D.T., Roccella E.J., Deutsch A.F., Fornage M., George M.G., Howard G., et al. Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke. 2014;45(1):315–353. doi: 10.1161/01.str.0000437068.30550.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chamandy N., Wolfson C. Underlying cause of death in demented and non-demented elderly Canadians. Neuroepidemiology. 2005;25(2):75–84. doi: 10.1159/000086287. [DOI] [PubMed] [Google Scholar]
- 3.Subic A., Cermakova P., Norrving B., Winblad B., von Euler M., Kramberger M.G., et al. Management of acute ischaemic stroke in patients with dementia. J. Intern. Med. 2017;281(4):348–364. doi: 10.1111/joim.12588. [DOI] [PubMed] [Google Scholar]
- 4.Cermakova P., Szummer K., Johnell K., Fastbom J., Winblad B., Eriksdotter M., et al. Management of acute myocardial infarction in patients with dementia: data from svedem, the swedish dementia registry. J. Am. Med. Dir. Assoc. 2017;18(1):19–23. doi: 10.1016/j.jamda.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 5.Secnik J., Cermakova P., Fereshtehnejad S.M., Dannberg P., Johnell K., Fastbom J., et al. Diabetes in a large dementia cohort: clinical characteristics and treatment from the swedish dementia registry. Diabetes Care. 2017;40(9):1159–1166. doi: 10.2337/dc16-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collaborators GMaCoD. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet S0140-6736(14): 61682-2 (2013) 2013 doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunnstrom H.R., Englund E.M. Cause of death in patients with dementia disorders. Eur. J. Neurol. 2009;16(4):488–492. doi: 10.1111/j.1468-1331.2008.02503.x. [DOI] [PubMed] [Google Scholar]
- 8.Kammoun S., Gold G., Bouras C., Giannakopoulos P., McGee W., Herrmann F., et al. Immediate causes of death of demented and non-demented elderly. Acta Neurol. Scand. Suppl. 2000;176:96–99. doi: 10.1034/j.1600-0404.2000.00314.x. [DOI] [PubMed] [Google Scholar]
- 9.Beard C.M., Kokmen E., Sigler C., Smith G.E., Petterson T., O’Brien P.C. Cause of death in Alzheimer’s disease. Ann. Epidemiol. 1996;6(3):195–200. doi: 10.1016/1047-2797(95)00068-2. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Ptacek S., Kareholt I., Cermakova P., Rizzuto D., Religa D., Eriksdotter M. Causes of death according to death certificates in individuals with dementia: a cohort from the swedish dementia registry. J. Am. Geriatr. Soc. 2016;64(11):e137–e42. doi: 10.1111/jgs.14421. [DOI] [PubMed] [Google Scholar]
- 11.Kukull W.A., Brenner D.E., Speck C.E., Nochlin D., Bowen J., McCormick W., et al. Causes of death associated with Alzheimer disease: variation by level of cognitive impairment before death. J. Am. Geriatr. Soc. 1994;42(7):723–726. doi: 10.1111/j.1532-5415.1994.tb06531.x. [DOI] [PubMed] [Google Scholar]
- 12.Staekenborg S.S., Pijnenburg Y.A., Lemstra A.W., Scheltens P., Vd Flier W.M. Dementia and rapid mortality: who is at risk? J. Alzheimers Dis. 2016;53(1):135–142. doi: 10.3233/JAD-151063. [DOI] [PubMed] [Google Scholar]
- 13.Molsa P.K., Marttila R.J., Rinne U.K. Survival and cause of death in Alzheimer’s disease and multi-infarct dementia. Acta Neurol. Scand. 1986;74(2):103–107. doi: 10.1111/j.1600-0404.1986.tb04634.x. [DOI] [PubMed] [Google Scholar]
- 14.Brooke H.L., Talbäck M., Hörnblad J., Johansson L.A., Ludvigsson J.F., Druid H., et al. The Swedish cause of death register. Eur. J. Epidemiol. 2017;32(9):765–773. doi: 10.1007/s10654-017-0316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alpérovitch A., Bertrand M., Jougla E., Vidal J-S., Ducimetière P., Helmer C., et al. Do we really know the cause of death of the very old? Comparison between official mortality statistics and cohort study classification. Eur. J. Epidemiol. 2009;24(11):669. doi: 10.1007/s10654-009-9383-2. [DOI] [PubMed] [Google Scholar]
- 16.Anders E., Hans S., Kristin A., Kurt B., Lars Olov B., Lars Age J., et al. Accuracy of death certificates of cardiovascular disease in a community intervention in Sweden. Scand. J. Public Health. 2013;41(8):883–889. doi: 10.1177/1403494813499653. [DOI] [PubMed] [Google Scholar]
- 17.Asplund K., Sukhova M., Wester P., Stegmayr B., Riksstroke C. Diagnostic procedures, treatments, and outcomes in stroke patients admitted to different types of hospitals. Stroke. 2015;46(3):806–812. doi: 10.1161/STROKEAHA.114.007212. [DOI] [PubMed] [Google Scholar]
- 18.Religa D., Fereshtehnejad S.M., Cermakova P., Edlund A.K., Garcia-Ptacek S., Granqvist N., et al. SveDem, the swedish dementia registry - a tool for improving the quality of diagnostics, treatment and care of dementia patients in clinical practice. PLoS One. 2015;10(2):e0116538. doi: 10.1371/journal.pone.0116538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Organization W.H. http://apps.who.int/classifications/icd10/browse/2015/en
- 20.Asplund K., Hulter Asberg K., Norrving B., Stegmayr B., Terent A., Wester P.O., et al. Riks-stroke - a Swedish national quality register for stroke care. Cerebrovasc. Dis. 2003;15(1):5–7. doi: 10.1159/000068203. [DOI] [PubMed] [Google Scholar]
- 21.Ludvigsson J.F., Andersson E., Ekbom A., Feychting M., Kim J.L., Reuterwall C., et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wettermark B., Hammar N., Fored C.M., Leimanis A., Otterblad Olausson P., Bergman U., et al. The new Swedish Prescribed Drug Register-opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol. Drug Saf. 2007;16(7):726–735. doi: 10.1002/pds.1294. [DOI] [PubMed] [Google Scholar]
- 23.Feigin V.L., Krishnamurthi R.V., Parmar P., Norrving B., Mensah G.A., Bennett D.A., et al. Update on the global burden of ischemic and hemorrhagic stroke in 1990-2013: the GBD 2013 study. Neuroepidemiology. 2015;45(3):161–176. doi: 10.1159/000441085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vernino S., Brown R.D., Jr, Sejvar J.J., Sicks J.D., Petty G.W., O’Fallon W.M. Cause-specific mortality after first cerebral infarction: a population-based study. Stroke. 2003;34(8):1828–1832. doi: 10.1161/01.STR.0000080534.98416.A0. [DOI] [PubMed] [Google Scholar]
- 25.Bronnum-Hansen H., Davidsen M., Thorvaldsen P., Danish M.S.G. Long-term survival and causes of death after stroke. Stroke. 2001;32(9):2131–2136. doi: 10.1161/hs0901.094253. [DOI] [PubMed] [Google Scholar]
- 26.Hartmann A., Rundek T., Mast H., Paik M.C., Boden-Albala B., Mohr J.P., et al. Mortality and causes of death after first ischemic stroke: the Northern Manhattan Stroke Study. Neurology. 2001;57(11):2000–2005. doi: 10.1212/wnl.57.11.2000. [DOI] [PubMed] [Google Scholar]
- 27.Moulin S., Leys D. Stroke occurring in patients with cognitive impairment or dementia. Arq. Neuropsiquiatr. 2017;75(2):117–121. doi: 10.1590/0004-282X20160187. [DOI] [PubMed] [Google Scholar]
- 28.Lakkireddy D.R., Gowda M.S., Murray C.W., Basarakodu K.R., Vacek J.L. Death certificate completion: how well are physicians trained and are cardiovascular causes overstated? Am. J. Med. 2004;117(7):492–498. doi: 10.1016/j.amjmed.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 29.Lenfant C., Friedman L., Thom T. Fifty years of death certificates: the Framingham Heart Study. Ann. Intern. Med. 1998;129(12):1066–1067. doi: 10.7326/0003-4819-129-12-199812150-00013. [DOI] [PubMed] [Google Scholar]
- 30.Corwin L.E., Wolf P.A., Kannel W.B., McNamara P.M. Accuracy of death certification of stroke: the Framingham Study. Stroke. 1982;13(6):818–821. doi: 10.1161/01.str.13.6.818. [DOI] [PubMed] [Google Scholar]
- 31.Hallstrom B., Jonsson A.C., Nerbrand C., Norrving B., Lindgren A. Stroke incidence and survival in the beginning of the 21st century in southern Sweden: comparisons with the late 20th century and projections into the future. Stroke. 2008;39(1):10–15. doi: 10.1161/STROKEAHA.107.491779. [DOI] [PubMed] [Google Scholar]
- 32.Sudlow C.L., Warlow C.P. Comparing stroke incidence worldwide: what makes studies comparable? Stroke. 1996;27(3):550–558. doi: 10.1161/01.str.27.3.550. [DOI] [PubMed] [Google Scholar]
- 33.Johansson L.A., Westerling R. Comparing Swedish hospital discharge records with death certificates: implications for mortality statistics. Intern J. 2000;29(3):495–502. [PubMed] [Google Scholar]
- 34.Welfare SBoHa. Nationella riktlinjer för vård vid stroke: Socialstyrelsen. 2017 [cited 2018 27.5.]. Available from: https://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/20618/2017-5-13.pdf.
- 35.Hallstrom B., Jonsson A.C., Nerbrand C., Petersen B., Norrving B., Lindgren A. Lund Stroke Register: hospitalization pattern and yield of different screening methods for first-ever stroke. Acta Neurol. Scand. 2007;115(1):49–54. doi: 10.1111/j.1600-0404.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- 36.Roman G.C., Tatemichi T.K., Erkinjuntti T., Cummings J.L., Masdeu J.C., Garcia J.H., et al. Vascular dementia: diagnostic criteria for research studies. Rep NINDS-AIREN Intern Workshop. Neurology. 1993;43(2):250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 37.Ferrucci L., Guralnik J.M., Salive M.E., Pahor M., Corti M.C., Baroni A., et al. Cognitive impairment and risk of stroke in the older population. J. Am. Geriatr. Soc. 1996;44(3):237–241. doi: 10.1111/j.1532-5415.1996.tb00908.x. [DOI] [PubMed] [Google Scholar]
- 38.Imfeld P., Bodmer M., Schuerch M., Jick S.S., Meier C.R. Risk of incident stroke in patients with Alzheimer disease or vascular dementia. Neurology. 2013;81(10):910–919. doi: 10.1212/WNL.0b013e3182a35151. [DOI] [PubMed] [Google Scholar]
- 39.Gorell J.M., Johnson C.C., Rybicki B.A. Parkinson’s disease and its comorbid disorders: an analysis of Michigan mortality data, 1970 to 1990. Neurology. 1994;44(10):1865–1868. doi: 10.1212/wnl.44.10.1865. [DOI] [PubMed] [Google Scholar]
- 40.Struck L.K., Rodnitzky R.L., Dobson J.K. Stroke and its modification in Parkinson’s disease. Stroke. 1990;21(10):1395–1399. doi: 10.1161/01.str.21.10.1395. [DOI] [PubMed] [Google Scholar]
- 41.Levine R.L., Jones J.C., Bee N. Stroke and Parkinson’s disease. Stroke. 1992;23(6):839–842. doi: 10.1161/01.str.23.6.839. [DOI] [PubMed] [Google Scholar]
- 42.Hobson P., Meara J. Mortality and quality of death certification in a cohort of patients with Parkinson’s disease and matched controls in North Wales, UK at 18 years: a community-based cohort study. BMJ Open. 2018;8(2):e018969. doi: 10.1136/bmjopen-2017-018969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fereshtehnejad S.M., Damangir S., Cermakova P., Aarsland D., Eriksdotter M., Religa D. Comorbidity profile in dementia with Lewy bodies versus Alzheimer’s disease: a linkage study between the Swedish Dementia Registry and the Swedish National Patient Registry. Alzheimers Res. Ther. 2014;6(5-8):65. doi: 10.1186/s13195-014-0065-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jellinger K.A. Prevalence of vascular lesions in dementia with Lewy bodies. A postmortem study. J. Neural Transm. (Vienna) 2003;110(7):771–778. doi: 10.1007/s00702-003-0824-x. [DOI] [PubMed] [Google Scholar]
- 45.Kalkonde Y.V., Jawaid A., Qureshi S.U., Shirani P., Wheaton M., Pinto-Patarroyo G.P., et al. Medical and environmental risk factors associated with frontotemporal dementia: a case-control study in a veteran population. Alzheimers Dement. 2012;8(3):204–210. doi: 10.1016/j.jalz.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 46.Weich S., Pearce H.L., Croft P., Singh S., Crome I., Bashford J., et al. Effect of anxiolytic and hypnotic drug prescriptions on mortality hazards: retrospective cohort study. BMJ. 2014;348:g1996. doi: 10.1136/bmj.g1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wooltorton E. Risperidone (Risperdal): increased rate of cerebrovascular events in dementia trials. CMAJ. 2002;167(11):1269–1270. [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J.P., Gallego J.A., Robinson D.G., Malhotra A.K., Kane J.M., Correll C.U. Efficacy and safety of individual second-generation vs. first-generation antipsychotics in first-episode psychosis: a systematic review and meta-analysis. Int. J. Neuropsychopharmacol. 2013;16(6):1205–1218. doi: 10.1017/S1461145712001277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gill S.S., Bronskill S.E., Normand S.L., Anderson G.M., Sykora K., Lam K., et al. Antipsychotic drug use and mortality in older adults with dementia. Ann. Intern. Med. 2007;146(11):775–786. doi: 10.7326/0003-4819-146-11-200706050-00006. [DOI] [PubMed] [Google Scholar]
- 50.Mittal V., Kurup L., Williamson D., Muralee S., Tampi R.R. Risk of cerebrovascular adverse events and death in elderly patients with dementia when treated with antipsychotic medications: a literature review of evidence. Am. J. Alzheimers Dis. Other Demen. 2011;26(1):10–28. doi: 10.1177/1533317510390351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Norgaard A., Jensen-Dahm C., Gasse C., Hansen H.V., Waldemar G. Time trends in antipsychotic drug use in patients with dementia: a nationwide study. J. Alzheimers Dis. 2015 doi: 10.3233/JAD-150481. [DOI] [PubMed] [Google Scholar]
- 52.Nerius M., Johnell K., Garcia-Ptacek S., Eriksdotter M., Haenisch B., Doblhammer G. The impact of antipsychotic drugs on long-term care, nursing home admission and death among dementia patients. J. Gerontol. A Biol. Sci. Med. Sci. 2017 doi: 10.1093/gerona/glx239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Asplund K., Lundström S., Stegmayr B. End of life after stroke: a nationwide study of 42,502 deaths occurring within a year after stroke. Eur Stroke J. 2018;3(1):74–81. doi: 10.1177/2396987317736202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garcia-Ptacek S., Farahmand B., Kareholt I., Religa D., Cuadrado M.L., Eriksdotter M. Mortality risk after dementia diagnosis by dementia type and underlying factors: a cohort of 15,209 patients based on the swedish dementia registry. J. Alzheimers Dis. 2014;41(2):467–477. doi: 10.3233/JAD-131856. [DOI] [PubMed] [Google Scholar]
- 55.Department of Health and Human Services Centers for Disease Control and Prevention, National Center for Health Statistics. Physicians’ handbook on medical certification of death. Hyattsville, Maryland: Government Printing Office; 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s web site along with the published article.


