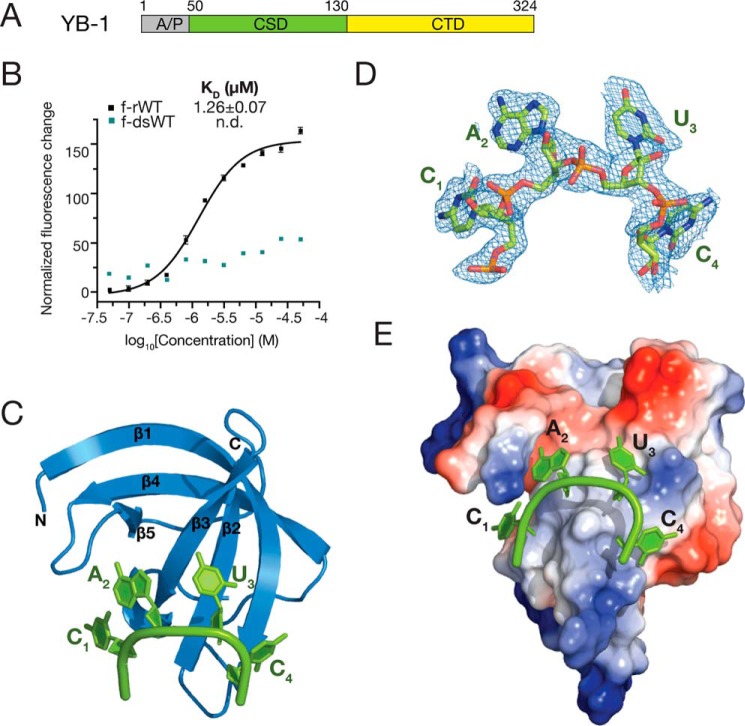
Figure 1.
Overall structure of YB-1 CSD in complex with CAUC. A, schematic representation of the domain architecture of human YB-1. B, interactions between YB-1 CSD and fluorescently labeled f-rWT or f-dsRNA oligos were analyzed by fluorescence polarization. One nanomolar of fluorescently labeled RNA was incubated with the protein at different concentrations. Changes in the fluorescence intensity changes under polarized illumination were plotted. Error bars, S.D. (n = 3). C, cartoon representation of the YB-1 CSD (blue) and CAUC (green) complex. YB-1 CSD represents a typical OB-fold, which is composed of five β-strands. D, 2Fo − Fc electron density map (contoured at 1.0 σ cutoff, blue mesh) of the tetranucleotide CAUC in the YB-1 CSD–CAUC structure. E, electrostatic potential surface analysis of the YB-1 CSD. RNA is bound to the hydrophobic and partially basic patch of the surface. A/P, Ala/Pro-rich domain; CTD, C-terminal domain.