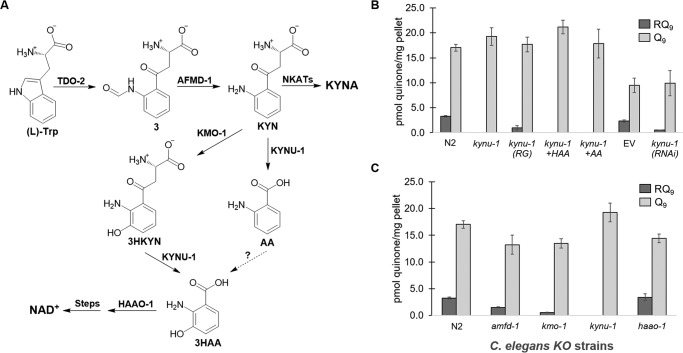
Figure 2.
Kynurenine pathway is essential for RQ biosynthesis. A, in the kynurenine pathway, l-tryptophan is first converted to l-formyl kynurenine (compound 3) by tryptophan 2,3-dioxygenase (TDO-2), which is then converted to KYN by an arylformamidase (AFMD-1). Kynurenine (KYN) is a branch point and can be converted to the following: 1) KYNA by kynurenine aminotransferases (NKATs); 2) 3HKYN by kynurenine 3-monooxygenase (KMO-1); or 3) AA by the kynureninase (KYNU-1). KYNU-1 also transforms 3HKYN to 3HAA, which has also been proposed to form from AA. Finally, 3HAA is converted to 2-amino-3-carboxymuconic semialdehyde (not shown) by 3-hydroxyanthranilic acid oxygenase (HAAO-1), which is then converted to NAD+. B, deletion of kynu-1 from N2 C. elegans abolished RQ biosynthesis. Overexpression of kynu-1 WT allele in the mutant kynu-1 strain restored RQ biosynthesis (RG). Supplementation with 3HAA and AA did not rescue RQ levels. The kynu-1 RNAi significantly reduced RQ levels compared with the EV control in rrf-3(pk1426) worms. C, amfd-1 and kymo-1 strains significantly reduced RQ levels, compared with N2, whereas the haao-1 strain had no effect. A full statistical analysis is given in Table S1.