FIGURE 4.
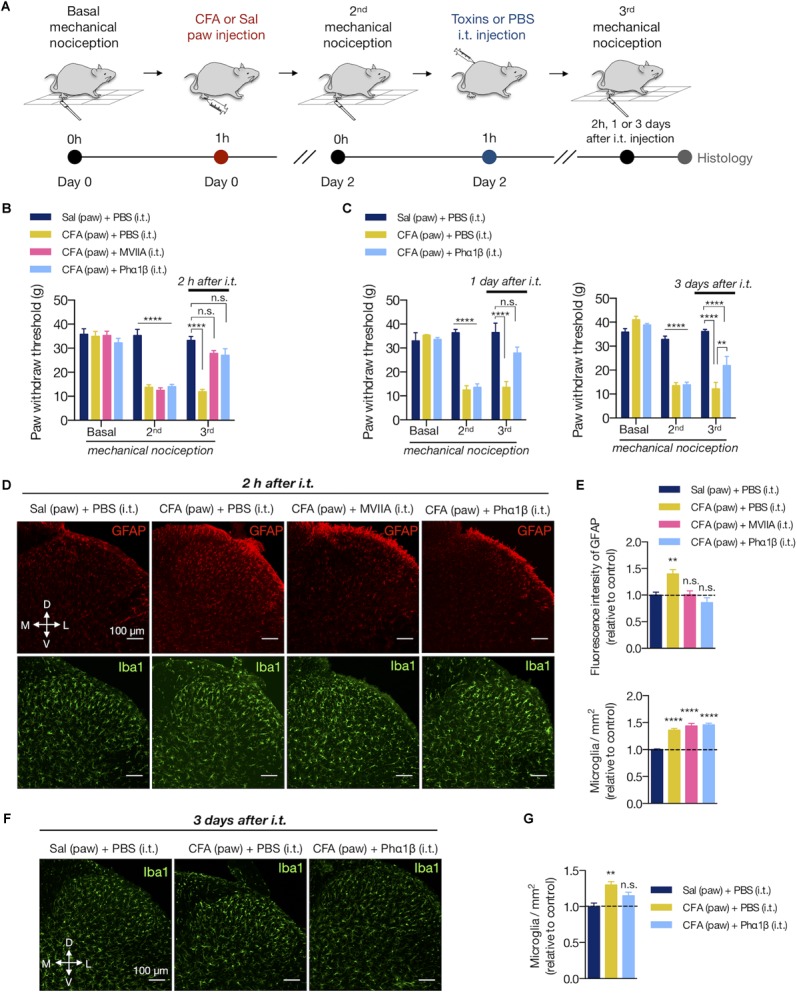
The calcium-channel blocker toxin Phα1β reverses allodynia and glial reactivity. (A) Experimental design for the CFA (complete Freund’s adjuvant) model of peripheral inflammatory pain followed by intrathecal (i.t) injections of PBS or calcium-channel blocker MVIIA and Phα1β toxins. After the basal mechanical nociception, CFA or saline was injected into the right paw (day 0). Two days later, the second (2nd) mechanical nociception was performed. Then, PBS or the MVIIA and Phα1β toxins were injected (i.t.). The third (3rd) mechanical nociception was performed 2 h, 1 or 3 days after the i.t. injection. Histology was carried out immediately after the 3rd mechanical nociception. (B) Mechanical nociception of the ipsilateral hind paw was quantified before (Basal, day 0), after 2 days of saline or CFA paw injection (2nd, day 2) and 2 h after the i.t. injections (MVIIA, Phα1β or PBS, 3rd, day 2) (n = 7–8/group). (C) In an independent group of animals, the 3rd mechanical nociception of the ipsilateral hind paw was also quantified 1 day (left) or 3 days (right) after the i.t injection of Phα1β or PBS (n = 4/group). (D) Representative fluorescence images and (E) quantification of the fluorescence intensity of GFAP (Stevens et al., 2007) or the number of microglia (Iba1+, in the bottom) in the ipsilateral dorsal horn of the lumbar spinal cord 2 h after the i.t. injection (n = 3–4/group, six slices per animal). Graphs show values relative to the control group [Sal (paw) + PBS (i.t)]. (F,G) In an independent group of animals, the number of microglia (Iba1+) was also quantified 3 days after the i.t injection of Phα1β or PBS (n = 4/group, three slices per animal). The values in the graph are relative to the control group [Sal (paw) + PBS (i.t)]. Scale bars are 100 μm (D,F). M: medial; L: lateral; D: dorsal; V: ventral (coordinates relative to the central canal). Summary data are represented as mean ± SEM. ∗∗p < 0.01; ∗∗∗∗p < 0.0001; n.s., not significant [compared to the Sal (paw) + PBS (i.t) group].
