Abstract
Study Objective
Dehydroepiandrosterone (DHEA) and its sulfated form (DHEAS)—jointly referred to as DHEA(S)—are neurosteroids known to regulate brain development and function that have been found to be positively correlated with cognitive function. It is unknown whether prechemotherapy plasma DHEA(S) levels are associated with the onset of cancer‐related cognitive impairment (CRCI). The objective of this study was to evaluate whether an association exists between prechemotherapy plasma DHEA(S) levels and onset of CRCI in patients with breast cancer receiving chemotherapy.
Design
Multicenter, prospective cohort study.
Setting
Two specialized cancer centers in Singapore.
Patients
Eighty‐one patients with early‐stage breast cancer (stages I–III) who had no prior exposure to chemotherapy and/or radiotherapy and were scheduled to receive anthracycline‐based or taxane‐based chemotherapy treatment with curative intent.
Measurements and Main Results
Patients completed assessments for self‐perceived and objective cognitive function at three time points: prechemotherapy (T1), during chemotherapy (T2), and after chemotherapy (T3). Plasma samples were collected prior to chemotherapy, and DHEA(S) levels were quantified by using ultra–high‐performance liquid chromatography–tandem mass spectrometry. Multivariable logistic regression was used to adjust for clinically important factors and to evaluate the association between prechemotherapy plasma DHEA(S) levels and CRCI. Mean ± SD age was 48.9 ± 9.3 years, with 27.8% of patients experiencing clinically significant cognitive impairment based on global Functional Assessment of Cancer Therapy–Cognitive Function scores. The mean ± SD prechemotherapy plasma DHEAS and DHEA levels were 1.61 ± 0.91 μmol/L and 19.21 ± 13.13 nmol/L, respectively. Prechemotherapy DHEAS levels were found to be associated with impairment in the self‐perceived cognitive domains of verbal fluency (adjusted odds ratio [OR] 0.27, 95% confidence interval [CI] 0.08–0.96) and mental acuity (adjusted OR 0.25, 95% CI 0.08–0.74). Conversely, DHEA levels were not associated with impairment in any cognitive subdomains.
Conclusion
Our findings suggest that patients with higher prechemotherapy DHEAS levels had lower odds of developing self‐perceived cognitive impairment. Future studies are required to further investigate the effect of DHEA(S) on specific cognitive domains and to validate our findings in independent cohorts.
Keywords: oncology, quality of life, chemotherapy, dehydroepiandrosterone, cancer‐related cognitive impairment
Although cancer has become more curable, with the use of chemotherapy leading to improved survival rates, many cancer survivors experience posttreatment adverse effects.1, 2 Of these adverse effects, cancer‐related cognitive impairment (CRCI) is an area of clinical concern that has been found to affect patients diagnosed with non–central nervous system cancer. CRCI is characterized as changes to mainly the cognitive domains of memory, processing speed, and executive function.3 In our previous studies, we observed postchemotherapy cognitive impairment in up to 37% of patients with breast cancer,4 and the effect persists in up to 30% of patients for as long as up to 15 months after treatment.5 Although the effects of CRCI may be transient or long term, cognitive changes can cause profound and negative impact on quality of life in patients with cancer.6
Given the extent of the impact of CRCI, there is a need to enhance our understanding of its underlying biological mechanisms. This will allow us to develop appropriate pharmacologic interventions, which are currently limited.7 There have been several proposed mechanisms of CRCI including, but not limited to, oxidative stress, dysregulation of inflammatory cytokines, genetic susceptibility, hormone deficiencies, and/or psychological distress,8, 9 although the cause of CRCI is likely to be multifactorial.
Dehydroepiandrosterone (DHEA) and its sulfated form (DHEAS)—jointly referred as DHEA(S)—are neurosteroids that serve important functions that help to regulate brain development, function, and behavior. DHEA(S) are purported to exert a cognitive‐preserving effect through their neuroprotective, antioxidant, antiinflammatory, and antiglucocorticoid actions.10 This is mediated by binding to brain receptors (such as the N‐methyl d‐aspartate and γ‐aminobutyric acid receptors) for which they have shown affinity or through the conversion into active metabolites or more potent steroid hormones such as estradiol.11 Although DHEA and DHEAS can be reversibly converted into each other, their molecular effects are not the same, and it is postulated that the balance between both forms influence brain functioning.12
In the literature, several studies have identified associations between baseline DHEA(S) levels and cognitive function, although the direction of the association may differ depending on the population studied and the measures used. In an epidemiologic cohort study, older women with higher plasma DHEA(S) levels were found to be at increased odds of reporting subjective cognitive concerns,13 whereas in patients with bipolar disorder, serum DHEAS levels were found to be inversely correlated with verbal memory.14 In contrast, male cancer survivors with lower DHEAS levels were found to perform worse on the attention measure in a cross‐sectional study.15 In population studies comprising the elderly, lower DHEA(S) level were associated with a higher prevalence of cognitive disorders in elderly patients diagnosed with Alzheimer's disease and depression,16 and baseline serum DHEAS levels remained positively associated with cognitive function during a 3‐year follow‐up period.17 From these studies, we hypothesized that lower DHEA(S) levels would be associated with poorer cognitive function in cancer survivors, given that they might be subjected to greater risk for cancer treatment‐related neurotoxicity.
The biological activity of DHEA(S) coupled with their association with cognitive function observed across various clinical populations lead us to suggest that DHEA(S) supplementation may potentially serve as a pharmacologic intervention to help with cognition in CRCI. Reports of DHEA(S) administration typically outline beneficial effects on cognition overall, with a certain specificity for working memory and attention,12, 18 which may support the use of DHEA(S) supplementation as a preemptive intervention to address CRCI. To support this statement, it would be prudent to know whether patients experiencing CRCI possess low levels of DHEA(S) prior to chemotherapy treatment.
Hence, the main objective of our study was to evaluate whether a relationship exists between prechemotherapy plasma DHEA(S) levels and CRCI in a cohort of patients with early‐stage breast cancer. If we can identify the characteristics of the patients who experienced CRCI with low baseline DHEA(S) levels, we can focus on this specific subgroup of patients for monitoring CRCI and tailor our intervention of DHEA(S) supplementation accordingly.
Methods
Study Design
This was a multicenter, prospective cohort study conducted in National Cancer Centre Singapore and KK Women's and Children's Hospital between 2011 and 2016. This study was approved by the Singhealth Institutional Review Board (IRB 2011/457/B), and written informed consent was obtained from all study patients.
The inclusion criteria were as follows: diagnosed with early‐stage breast cancer (stages I–III), had no prior exposure to chemotherapy and/or radiotherapy, scheduled to receive anthracycline‐based or taxane‐based chemotherapy treatment with curative intent, capable of providing informed consent, and understood either English or Chinese. Patients were excluded if they were diagnosed with neurologic or psychiatric medical conditions.
All study patients were assessed at three time points: before treatment initiation (baseline; T1), during chemotherapy, at least 6 weeks after baseline (T2), and after completion of chemotherapy, at least 12 weeks after baseline, (T3). Prior to chemotherapy initiation, each patient's demographic and clinical data were collected and verified through patient interviews and electronic medical records.
Study Tools
At each time point (T1, T2, and T3), patients completed a series of questionnaires to assess their cognitive function, behavioral symptoms, and quality of life. All assessments were administered by trained bilingual research assistants in either English or Chinese and took approximately 40 minutes to complete.
For self‐perceived cognitive function, the Functional Assessment of Cancer Therapy–Cognitive Function (FACT‐Cog) Version 3 is a validated questionnaire used to assess the impact on the patient's quality of life within the past 7 days.19 Items in the subscale were organized into the respective subdomains of mental acuity, concentration, memory, verbal fluency, functional interference, and multitasking.20 The responses were reverse‐scored in which higher scores indicated better self‐perceived cognitive function. Patients were defined as having self‐perceived cognitive impairment at each time point if there was a reduction of 10.6 on the global FACT‐Cog score relative to baseline (T2‐T1 or T3‐T1), based on the minimal clinically important difference (MCID) determined in patients with breast cancer.21 For each of the cognitive subdomain, a reduction of 15% in scores from baseline is considered as self‐perceived cognitive impairment. This was determined a priori, based on the method of approach from other studies that have used FACT‐Cog to evaluate perceived cognitive function.4, 5
For objective cognitive function, a computerized neuropsychological test, Headminder, was used to assess four domains: processing speed, response time, memory, and attention.22 In this study, patients were defined as having cognitive impairment at each time point for each cognitive domain if the Reliable Change Index (RCI) was lower than −1.5 relative to baseline (T2‐T1 or T3‐T1).3 The RCI accounted for practice effect and measurement error by considering the repeated normative mean and standard error of the difference.22
At each time point, the self‐administered Brief Fatigue Inventory (BFI), Beck Anxiety Inventory (BAI), and the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ‐C30) were also used to capture patients’ fatigue symptoms, anxiety symptoms, and health‐related quality of life, respectively. The symptom scale of the EORTC was used to assess for insomnia in our patient cohort.
Quantification of Prechemotherapy Plasma DHEA(S) Levels
Prior to initiation of chemotherapy, blood (10 ml) was collected from each patient and stored in ethylenediaminetetraacetic acid tubes. The blood sample was centrifuged at 1069 × g for 30 minutes, and the plasma sample was stored in a −80°C freezer until sample analysis. Ultra–high‐performance liquid chromatography–tandem mass spectrometry (UPLC‐MS/MS) methods were developed and quantitatively validated for specificity, linearity, lower limit of quantification (LLOQ), accuracy and precision, matrix effect, and extraction recovery in accordance with the United States Food and Drug Administration's Bioanalytical Method Validation (May 2018)23 for the quantification of DHEA(S) levels in patient plasma (Table S1).
The samples prepared were assayed using an in‐house developed UPLC‐MS/MS methodology. An Agilent C18 column (2.1 × 50 mm, 1.8 μm) (Agilent Technologies, Santa Clara, CA) was used in tandem with an AB Sciex 3500 triple quadrupole mass spectrometer (Applied Biosystems, Foster City, CA). Negative electrospray ionization (ESI) with multiple reaction monitoring mode was used to quantify DHEAS (367.0 → 97.0) whereas the positive ESI mode was used for DHEA (289.1 → 253.6) in their respective mass/charge ratios (m/z). Internal standards of estrone 3‐sulfate‐d5 sodium salt (354.2 → 274.0) and cortisol‐d4 (367.2 → 121.0) were used for DHEAS and DHEA, respectively. DHEA(S) levels were then determined by using Version 1.4.2 of Analyst Software (Applied Biosystems). The mobile phase consisted of (A) 10 mM ammonium acetate and (B) acetonitrile for DHEAS, and (A) 0.1% formic acid and (B) 0.1% formic acid in acetonitrile for DHEA.
Statistical Analysis
Descriptive statistics were used to summarize patients’ demographic and clinical characteristics. The number of patients with CRCI relative to baseline (T2‐T1 or T3‐T1) was described as proportions. An independent t test was used for subgroup analyses to compare DHEA(S) levels between patients who were cognitively impaired and nonimpaired, defined by the respective subdomains. Multivariable logistic regression was used to evaluate the association between prechemotherapy DHEA(S) levels and CRCI, with results reported as odd ratios (ORs) with 95% confidence intervals (CIs). Clinically important factors that may influence cognitive function, including age,24 years of education,25 fatigue at baseline,26 anxiety at baseline,27 insomnia at baseline,28 planned chemotherapy regimen,29 and menopausal status,30 were adjusted for in the final model. All statistical analyses were performed with STATA version 15 (2017) (StataCorp, College Station, TX), and two‐sided p values less than 0.05 were considered to indicate a statistically significant difference.
Results
Patient Characteristics
A total of 81 patients were included in the analysis (Figure 1), and their baseline demographic and clinical characteristics are summarized in Table 1. Mean ± SD age and years of education of the patients were 48.9 ± 9.3 and 11.0 ± 3.4 years, respectively. The patients were predominantly Chinese (82.7%), had stage II breast cancer (54.3%), and had excellent performance status (ECOG = 0, 91.4%). More than half of the patients were receiving an anthracycline‐based chemotherapy regimen (66.7%) and were premenopausal (53.1%).
Figure 1.
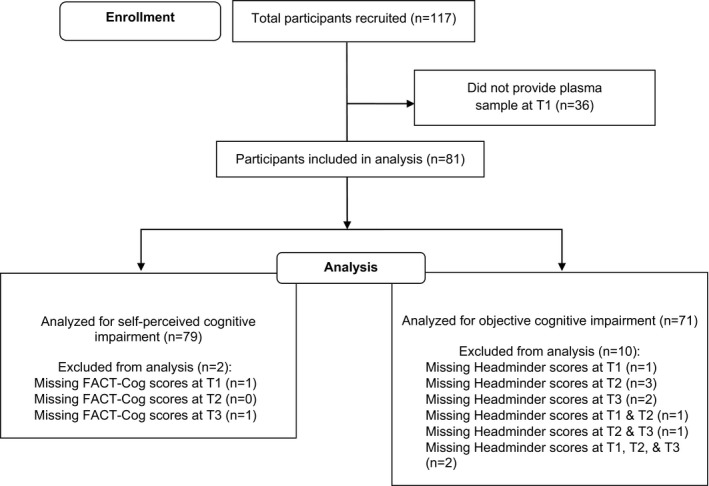
Flow diagram of the patient selection process. FACT‐Cog = Functional Assessment of Cancer Therapy–Cognitive Function.
Table 1.
Baseline Demographic and Clinical Characteristics of the Study Patients
| Characteristic | Data (n=81) |
|---|---|
| Age (yrs) | 48.9 ± 9.3 |
| Years of education | 11.0 ± 3.4 |
| DHEAS level (μmol/L) | 1.61 ± 0.91 |
| DHEA level (nmol/L) | 19.21 ± 13.13 |
| Fatigue level scorea | 1.76 ± 1.69 |
| Anxiety level scoreb | 7.27 ± 6.38 |
| Insomnia scorec | 20.25 ± 25.82 |
| Ethnicity | |
| Chinese | 67 (82.7) |
| Malay | 9 (11.1) |
| Indian | 2 (2.5) |
| Other | 3 (3.7) |
| Breast cancer stage | |
| I | 14 (17.3) |
| II | 44 (54.3) |
| III | 23 (28.4) |
| ECOG performance status | |
| 0 | 74 (91.4) |
| 1 | 7 (8.6) |
| Chemotherapy regimen | |
| Anthracycline based | 54 (66.7) |
| Taxane based | 27 (33.3) |
| Menopausal status | |
| Premenopausal | 43 (53.1) |
| Postmenopausal | 38 (46.9) |
Data are mean ± SD values or no. (%) of patients.
DHEA = dehydroepiandrosterone; DHEAS = DHEA sulfate; ECOG = Eastern Cooperative Oncology Group.
The Brief Fatigue Inventory's total score is out of a maximum of 10 points, with a higher score indicating more severe fatigue.
The Beck Anxiety Inventory's total score is out of a maximum of 63 points, with a higher score indicating more severe anxiety.
The insomnia's subscale total score is out of a maximum of 100 points, with a higher score indicating more or worse symptoms.
Overall Rate of Self‐Perceived and Objective CRCI
The mean ± SD for global FACT‐Cog scores across the various time points were as follows: 116.7 ± 15.0 (T1), 118.2 ± 14.2 (T2), and 114.0 ± 15.0 (T3). (Figure S1) Based on global FACT‐Cog scores, 13.8% and 21.5% of patients reported self‐perceived cognitive impairment at T2 and T3, respectively. The overall percentage of patients experiencing self‐perceived cognitive impairment over the study period was 27.8% (Table 2). For self‐perceived cognitive impairment, the proportion of patients (n=79) impaired in mental acuity (24.1%), concentration (21.5%), memory (13.9%), verbal fluency (12.7%), functional interference (13.9%) and multitasking (26.6%) cognitive domains are as listed respectively. With regard to objective cognitive impairment with the assessments conducted using Headminder, the proportion of patients (n=71) impaired in memory (21.1%), attention (12.7%), processing speed (7.0%), and response speed (5.6%) are as listed respectively.
Table 2.
Frequency of Self‐Perceived and Objective Cancer‐Related Cognitive Impairment
| No. (%) of Patientsa | |||
|---|---|---|---|
| T2‐T1 | T3‐T1 | Overall | |
| Self‐perceived cognitive function, FACT‐Cog Domainsb | (n=80) | (n=79) | (n=79) |
| Total FACT‐Cog score | 11 (13.8) | 17 (21.5) | 22 (27.8) |
| Mental acuity | 11 (13.8) | 15 (19.0) | 19 (24.1) |
| Concentration | 10 (12.5) | 12 (15.2) | 17 (21.5) |
| Memory | 6 (7.5) | 9 (11.4) | 11 (13.9) |
| Verbal fluency | 5 (6.3) | 7 (8.9) | 10 (12.7) |
| Functional Interference | 4 (5.0) | 7 (8.9) | 11 (13.9) |
| Multitasking | 12 (15.0) | 15 (19.0) | 21 (26.6) |
| Objective cognitive function, Headminder domainsc | (n=78) | (n=78) | (n=71) |
| Processing speed | 2 (2.6) | 4 (5.2) | 5 (7.0) |
| Response speed | 2 (2.6) | 2 (2.6) | 4 (5.6) |
| Memory | 7 (9.0) | 13 (16.7) | 15 (21.1) |
| Attention | 8 (6.0) | 4 (5.2) | 9 (12.7) |
FACT‐Cog = Functional Assessment of Cancer Therapy–Cognitive Function.
Patients were excluded from the analysis for a particular domain if they had missing measures at T1 and/or the measured time point (T2 or T3, respectively).
Self‐perceived cognitive impairment was defined as any decrease in FACT‐Cog global score of 10.6 points or more, whereas self‐perceived cognitive impairment in specific domains was defined as any 15% decrease from baseline scores.
Objective cognitive impairment was defined as a reliable change index score < −1.5 compared with baseline in the particular Headminder domain.
Comparison of Prechemotherapy DHEA(S) Levels across Respective Cognitive Subdomains
The mean ± SD plasma DHEAS and DHEA levels were reported to be 1.61 ± 0.91 μmol/L and 19.21 ± 13.13 nmol/L, respectively. DHEA(S) levels were compared and analyzed across the various cognitive subdomains (Table 3). In the domain of verbal fluency, DHEAS levels were significantly lower in the cognitively impaired group compared with the non–cognitively impaired group (0.98 ± 0.43 μmol/L vs 1.70 ± 0.94 μmol/L, p=0.02) (Figure 2). Lower DHEAS levels were observed in patients with cognitive impairment for majority of the identified cognitive subdomains except for the domain of attention. Similarly, lower absolute plasma DHEA levels were also observed in cognitively impaired patients defined by mental acuity, concentration, verbal fluency, multitasking, and objective domains of processing speed. However, no statistically significant differences between the two groups were found.
Table 3.
Prechemotherapy Plasma DHEAS and DHEA Levels in Patients in the Cognitively Impaired vs Non–Cognitively Impaired Groups Defined by their Respective Subdomains
| Cognitive Measure | DHEAS Level | DHEA Level | ||||
|---|---|---|---|---|---|---|
| Cognitively Impaired Group (μmol/L) | Non‐Cognitively impaired group (μmol/L) | p Value | Cognitively Impaired Group (nmol/L) | Non–Cognitively Impaired Group (nmol/L) | p Value | |
| Self‐perceived cognitive measurea | ||||||
| FACT‐Cog global score | 1.37 ± 0.15 (n=22) | 1.69 ± 0.13 (n=57) | 0.16 | 19.14 ± 9.07 (n=22) | 19.07 ± 14.41 (n=57) | 0.98 |
| Mental acuity | 1.27 ± 0.15 (n=19) | 1.71 ± 0.13 (n=60) | 0.07 | 18.03 ± 9.46 (n=19) | 19.43 ± 14.09 (n=60) | 0.68 |
| Concentration | 1.30 ± 0.68 (n=17) | 1.69 ± 0.96 (n=62) | 0.11 | 18.99 ± 9.47 (n=17) | 19.12 ± 13.98 (n=62) | 0.97 |
| Memory | 1.24 ± 0.73 (n=11) | 1.66 ± 0.94 (n=68) | 0.17 | 20.92 ± 11.09 (n=11) | 18.80 ± 13.43 (n=68) | 0.62 |
| Verbal fluency | 0.98 ± 0.43 (n=10) | 1.70 ± 0.94 (n=69) | 0.02 | 16.52 ± 5.86 (n=10) | 19.47 ± 13.81 (n=69) | 0.51 |
| Functional interference | 1.28 ± 0.64 (n=11) | 1.66 ± 0.95 (n=68) | 0.21 | 20.27 ± 10.25 (n=11) | 18.91 ± 13.54 (n=68) | 0.75 |
| Multitasking | 1.57 ± 0.82 (n=21) | 1.61 ± 0.96 (n=58) | 0.83 | 18.46 ± 8.05 (n=21) | 19.32 ± 14.54 (n=58) | 0.79 |
| Objective cognitive measureb | ||||||
| Processing speed | 1.09 ± 0.55 (n=5) | 1.73 ± 0.94 (n=66) | 0.13 | 14.62 ± 3.00 (n=5) | 19.55 ± 13.65 (n=66) | 0.42 |
| Response speed | 1.33 ± 0.76 (n=4) | 1.70 ± 0.93 (n=67) | 0.43 | 23.59 ± 12.27 (n=4) | 18.94 ± 13.33 (n=67) | 0.49 |
| Memory | 1.65 ± 0.85 (n=15) | 1.70 ± 0.95 (n=56) | 0.84 | 21.62 ± 8.37 (n=15) | 18.55 ± 14.25 (n=56) | 0.43 |
| Attention | 1.90 ± 0.61 (n=9) | 1.66 ± 0.96 (n=62) | 0.47 | 20.04 ± 14.22 (n=9) | 19.10 ± 13.21 (n=62) | 0.83 |
Bolded values indicate statistical significance.
Data are mean ± SD values.
DHEA = dehydroepiandrosterone; DHEAS = DHEA sulfate; FACT‐Cog = Functional Assessment of Cancer Therapy–Cognitive Function.
Self‐perceived cognitive impairment was defined as any decrease in FACT‐Cog global score of 10.6 points or more, whereas self‐perceived cognitive impairment in specific domains was defined as any 15% decrease from baseline scores.
Objective cognitive impairment was defined as a reliable change index score < −1.5 compared with baseline in the particular Headminder domain.
Figure 2.

Box plots of mean ± SD baseline plasma dehydroepiandrosterone sulfate (DHEAS) levels for the non–cognitively impaired and cognitively impaired groups defined by the verbal fluency domain.
Association between Prechemotherapy DHEA(S) Levels and Clinically Relevant Factors and CRCI
In terms of self‐perceived cognitive impairment, DHEAS level was associated with the domain of verbal fluency (OR 0.29, 95% CI 0.09–0.88). DHEA was not associated with any self‐perceived cognitive domains (both unadjusted and adjusted ORs), with its ORs being mostly close to 1 (Table 4). After adjusting for age, years of education, fatigue level, anxiety level, insomnia, type of chemotherapy regimen, and menopausal status, the association between prechemotherapy plasma DHEAS levels and verbal fluency was retained (adjusted OR 0.27, 95% CI 0.08–0.96). This adjusted OR of 0.27 meant that for every unit increase in prechemotherapy plasma DHEAS level (μmol/L), there was a 73% less risk of being cognitively impaired defined by verbal fluency during the period of chemotherapy, after adjusting for the listed confounders. In addition, DHEAS level was associated with impairment in the self‐perceived cognitive domain of mental acuity (adjusted OR 0.25, 95% CI = 0.08–0.74) after adjusting for confounders.
Table 4.
Association between Prechemotherapy Plasma DHEAS and DHEA Levels with Onset of Cancer‐Related Cognitive Impairment during Chemotherapy
| Cognitive Measure | DHEAS Level | DHEA Level | ||
|---|---|---|---|---|
| OR (95% CI) | Adjusted OR (95% CI)a | OR (95% CI) | Adjusted OR (95% CI)a | |
| Self‐perceived cognitive measure | ||||
| FACT‐Cog global score | 0.65 (0.36–1.19) | 0.48 (0.23–0.99) | 1.00 (0.96–1.03) | 1.00 (0.95–1.05) |
| Mental acuity | 0.55 (0.28–1.07) | 0.25 (0.08–0.74) | 0.99 (0.95–1.03) | 0.99 (0.94–1.06) |
| Concentration | 0.58 (0.29–1.15) | 0.45 (0.20–1.00) | 0.99 (0.96–1.04) | 1.00 (0.95–1.06) |
| Memory | 0.56 (0.24–1.28) | 0.64 (0.24–1.67) | 1.01 (0.97–1.06) | 1.02 (0.95–1.09) |
| Verbal fluency | 0.29 (0.09–0.88) | 0.27 (0.08–0.96) | 0.98 (0.92–1.04) | 0.97 (0.89–1.05) |
| Functional interference | 0.59 (0.26–1.35) | 0.55 (0.20–1.50) | 1.00 (0.96–1.06) | 1.04 (0.97–1.10) |
| Multitasking | 0.94 (0.54–1.63) | 0.89 (0.45–1.76) | 0.99 (0.96–1.03) | 0.98 (0.93–1.04) |
| Objective cognitive measure | ||||
| Processing speed | 0.36 (0.09–1.42) | 0.15 (0.01–1.78) | 0.96 (0.87–1.06) | 0.90 (0.73–1.11) |
| Response speed | 0.60 (0.17–2.17) | 0.09 (0.004–2.04) | 1.02 (0.96–1.08) | 1.10 (0.96–1.28) |
| Memory | 0.94 (0.50–1.77) | 1.00 (0.49–2.06) | 1.02 (0.97–1.06) | 1.01 (0.96–1.06) |
| Attention | 1.33 (0.64–2.76) | 1.53 (0.53–4.43) | 1.00 (0.96–1.06) | 1.03 (0.97–1.10) |
Bolded values indicate statistical significance.
CI = confidence interval; DHEA = dehydroepiandrosterone; DHEAS = DHEA sulfate; FACT‐Cog = Functional Assessment of Cancer Therapy–Cognitive Function; OR = odds ratio.
Adjusted for age, years of education, fatigue level, anxiety level, insomnia, type of chemotherapy regimen, and menopausal status.
There were no statistically significant association between DHEA(S) levels and impairment in the objective cognitive measures of processing speed, response speed, memory, and attention. In the univariate analysis, age, fatigue, and anxiety levels were found to be significantly associated with objective cognitive impairment (Table S2). With respect to predictors of onset of objective cognitive impairment, older age was found to be associated with higher odds of developing CRCI in the domain of attention (OR 1.10, 95% CI 1.00–1.21), whereas greater fatigue levels and anxiety levels were found to be associated with domain of response speed (OR 1.77, 95% CI 1.02–3.07, and OR 1.13, 95% CI 1.00–1.29, respectively). In contrast, none of the confounders were found to be statistically significant in impairment in the self‐perceived cognitive domains.
Discussion
This study aimed to investigate the association between prechemotherapy plasma DHEA(S) levels with CRCI, with our hypothesis being that lower DHEA(S) levels would be associated with poorer cognitive function. Consistent with our hypothesis, higher prechemotherapy DHEAS levels were reported to be associated with lower odds of developing cognitive impairment in the self‐perceived domains of verbal fluency (adjusted OR 0.27, 95% CI 0.08–0.96) and mental acuity (adjusted OR 0.25, 95% CI 0.08–0.74) after adjusting for confounders. The association observed with verbal fluency is corroborated by findings from a review that identified verbal fluency, working memory, and attention as domains with which DHEAS showed positive correlation.18 However, we did not observe higher DHEAS levels being favorably associated with executive function, concentration, and working memory in our cohort, which were domains identified in a community‐based study conducted in women.31 More studies are required to further investigate the effect of DHEAS level on specific cognitive domains. Conversely, prechemotherapy DHEA levels do not predict for onset of CRCI given how their reported ORs were mostly close to 1, which translated to a little or no change in cognitive status per unit increase in prechemotherapy plasma levels. There does not seem to be a major role contributed by DHEA(S) level in pathways related to objective cognitive impairment, with none of the associations being identified as statistically significant.
In terms of clinically relevant factors, age, fatigue level, and anxiety level were found to be related to onset of objective CRCI in our cohort. This finding is congruent with a review that identified that risk factors for CRCI included cognitive reserve, age, genetic factors, and ethnicity1 and a study that reported that older age was associated with a higher odds of developing CRCI in the domain of attention.32 There are also findings that suggest lower baseline serum DHEAS levels being associated with physical frailty and related adverse health outcomes in the elderly.33 In another study assessing clinical factors that correlate with baseline cognitive function of patients with breast cancer, age was similarly identified to be a predictor, with up to 40% of elderly patients with breast cancer presenting with cognitive deficits even before any adjuvant therapy.34 In addition, anxiety, depression, and fatigue levels were also found to be correlated with cognitive complaint scores. It should be noted that most studies that observed positive associations between plasma DHEA(S) levels and cognition were conducted in the elderly population, and this correlation could have been possibly mediated by age‐related decline of plasma DHEA(S) levels. These studies in the elderly have found a positive association with plasma DHEAS levels, with the listed domains of functional interference,35, 36 processing speed,37 and memory.18
There could be several possible reasons to explain why there was a lack of association observed between DHEA levels and other cognitive subdomains. Although DHEA(S) are related along the same pathway, with each being able to reversibly convert to the other, prechemotherapy plasma DHEA(S) levels have varying influence on the onset of CRCI. This could be related to the location of brain region affected by CRCI, with the hippocampus being the most likely, since decline in memory is maintained by the prefrontal cortex and hippocampus.38 Neuroendocrinological studies have shown that the concentrations of DHEA(S) across different brain regions vary considerably, with DHEA showing the highest concentration in the prefrontal cortex,11 and DHEAS being mostly concentrated at the posterior region of the brain.39 The lack of association seen for DHEA may also be attributed to a larger variation in plasma DHEA levels compared to DHEAS due to DHEA's relatively short half‐life40 as well as the diurnal41 and/or menstrual cycle variation.42 Given how levels of DHEAS may be indicative of hypothalamic‐pituitary‐adrenal (HPA) axis integrity, the greater extent of the impact observed with DHEAS also suggests that the dysfunction of HPA axis and/or impairment of adrenal function may potentially contribute to poorer neurocognitive outcomes among cancer survivors.43 Together, the difference in pharmacokinetic profiles, location, and choice of medium contribute as factors that could have undermined the magnitude of association between DHEA levels and CRCI.
Being able to establish a relationship between prechemotherapy plasma levels of DHEAS with impairment in specific cognitive domains would be useful in a clinical setting. There is potential application for the use of DHEA(S) supplementation as replacement therapy to mitigate the impact of CRCI in subgroup of patients who have low prechemotherapy levels to begin with, and who might be more vulnerable to its effects. DHEA(S) supplements are currently widely available, but the efficacy and adverse effect profile of DHEA(S) supplementation have not been demonstrated in many clinical studies.16, 44 The pathway‐related actions may be dependent on the type of population, be it clinical, elderly individuals, or healthy controls. In fact, an opposing review surmised that in studies with DHEA supplementation, improvement in cognitive function was not achieved.18 This begs the question that the lack of biological activity of DHEA(S) on the site of action could be attributed to poor oral bioavailability or insufficient doses.45 DHEA is typically marketed as a dietary supplement in doses of 25–50 mg per day but doses up to 300 mg may be chosen for clinical studies. Although there is general agreement that DHEA(S) supplementation can exert beneficial effects on mood and well‐being,12, 18 robust clinical trials need to be conducted before it can be determined whether DHEA(S) supplementation may serve as a potential pharmacologic intervention for CRCI.
Compared to other studies in literature investigating CRCI, our study's strength stems from its longitudinal nature, which allows us to discern the different trajectories of CRCI over time. In addition, we have distinguished and evaluated both objective and subjective assessments of cognitive changes in our patient cohort. As objective assessments evaluate the patient's cognitive function in a controlled setting, they often fail to consider patients’ experience with activities of daily living and the impact on quality of life.46, 47
This study has several limitations. First, measurement of plasma DHEA(S) level may not be indicative of actual physiologic levels in the site of action—brain tissue. However, obtaining tissue samples and cerebrospinal fluid would be invasive and logistically challenging, and thus plasma would present the most suited medium. The brain‐to‐plasma ratios for DHEA and DHEAS have been found to be about 4–6.5 and 8.5, respectively, with higher concentrations of neurosteroid found in the brain, although wide interindividual differences have also been observed.48 Second, although DHEA supplementation may improve memory, it is unclear whether this effect could have been mediated through conversion of these steroids into androgens or estradiol. Future work should also account for levels of sex hormones and patients who are taking hormonal replacement therapies in order to demonstrate a clearer correlation with cognition. Third, the International Cognition and Cancer Task Force recommends including diseased and/or healthy controls in studies.3 The addition of a diseased control group—non–chemotherapy‐receiving patients with breast cancer—may provide more information on whether lower DHEA(S) levels could be due to the malignancy itself. Lastly, we also acknowledge the lack of adjustment for multiple testing for each of the specific subdomains of cognition in our data analysis. Given the exploratory nature of our study, our findings in relation to subdomains may be considered as hypothesis generating until these findings have been validated in independent patient cohorts.
Conclusion
Our findings suggest that patients with higher prechemotherapy plasma levels of DHEAS have lower odds of developing self‐perceived cognitive impairment in verbal fluency and mental acuity. Future studies are required to further investigate the effect of DHEA(S) on specific cognitive domains and to validate our findings in independent cohorts. More research should also be conducted to understand role of DHEA(S) as predictors of cognitive function in pathways related to CRCI.
Supporting information
Table S1. Development and validation for the methodology for quantitative determination of plasma DHEA(S) levels.
Table S2A. Association of DHEA(S) and clinically relevant factors with self‐perceived CRCI.
Table S2B. Association of DHEA(S) and clinically relevant factors with objective CRCI.
Figure S1. (A) Graph plot of global FACT‐Cog scores against time points for cognitively impaired patients. (B). Graph plot of global FACT‐Cog scores against time points for non–cognitively impaired patients.
Acknowledgments
This work was supported by research grants awarded by the National University of Singapore (R‐148‐000‐233‐114; principal investigator Alex Chan), National Cancer Centre Singapore (NRFCB12131; principal investigator Alex Chan), and National Medical Research Council (NMRC/CIRG/1386/2014 and NMRC/CIRG/1471/2017; primary investigator Alex Chan).
Conflict of interest: The authors have declared no conflicts of interest for this article.
References
- 1. Hardy SJ, Krull KR, Wefel JS, Janelsins M. Cognitive changes in cancer survivors. Am Soc Clin Oncol Educ Book 2018;23(38):795–806. [DOI] [PubMed] [Google Scholar]
- 2. Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer‐related cognitive impairment. Int Rev Psychiatry 2014;26(1):102–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wefel JS, Vardy J, Ahles T, Schagen SB. International cognition and cancer task force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol 2011;12(7):703–8. [DOI] [PubMed] [Google Scholar]
- 4. Ng T, Teo SM, Yeo HL, et al. Brain‐derived neurotrophic factor genetic polymorphism (rs6265) is protective against chemotherapy‐associated cognitive impairment in patients with early‐stage breast cancer. Neuro Oncol 2016;18(2):244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ng T, Dorajoo SR, Cheung YT, et al. Distinct and heterogeneous trajectories of self‐perceived cognitive impairment among Asian breast cancer survivors. Psychooncology 2018;27(4):1185–92. [DOI] [PubMed] [Google Scholar]
- 6. Janelsins MC, Heckler CE, Peppone LJ, et al. Longitudinal trajectory and characterization of cancer‐related cognitive impairment in a Nationwide Cohort Study. J Clin Oncol 2018;36:3231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chan RJ, McCarthy AL, Devenish J, Sullivan KA, Chan A. Systematic review of pharmacologic and non‐pharmacologic interventions to manage cognitive alterations after chemotherapy for breast cancer. Eur J Cancer 2015;51(4):437–50. [DOI] [PubMed] [Google Scholar]
- 8. Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy‐induced cognitive changes. Nat Rev Cancer 2007;7(3):192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Merriman JD, Von Ah D, Miaskowski C, Aouizerat BE. Proposed mechanisms for cancer‐ and treatment‐related cognitive changes. Semin Oncol Nurs 2013;29(4):260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maggio M, De Vita F, Fisichella A, et al. DHEA and cognitive function in the elderly. J Steroid Biochem Mol Biol 2015;145:281–92. [DOI] [PubMed] [Google Scholar]
- 11. Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Neuroendocrinol 2009;30(1):65–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. do Vale S, Selinger L, Martins JM, Bicho M, do Carmo I, Escera C. Dehydroepiandrosterone (DHEA) and dehydroepiandrosterone‐sulfate (DHEAS) and emotional processing – a behavioral and electrophysiological approach. Horm Behav 2015;73:94–103. [DOI] [PubMed] [Google Scholar]
- 13. Koyama AK, Tworoger SS, Eliassen AH, et al. Endogenous sex hormones and cognitive function in older women. Alzheimers Dement 2016;12(7):758–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee SY, Wang LJ, Chang CH, et al. Serum DHEA‐S concentration correlates with clinical symptoms and neurocognitive function in patients with bipolar II disorder: a case‐controlled study. Prog Neuropsychopharmacol Biol Psychiatry 2017;74:31–5. [DOI] [PubMed] [Google Scholar]
- 15. Cheung YT, Chemaitilly W, Mulrooney DA, et al. Association between dehydroepiandrosterone‐sulfate and attention in long‐term survivors of childhood acute lymphoblastic leukemia treated with only chemotherapy. Psychoneuroendocrinology 2017;76:114–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sorwell KG, Urbanski HF. Dehydroepiandrosterone and age‐related cognitive decline. Age 2010;32(1):61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Valenti G, Ferrucci L, Lauretani F, et al. Dehydroepiandrosterone sulfate and cognitive function in the elderly: The InCHIANTI Study. J Endocrinol Invest 2009;32(9):766–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Menezes KJ, Peixoto C, Nardi AE, Carta MG, Machado S, Veras AB. Dehydroepiandrosterone, its sulfate and cognitive functions. Clin Pract Epidemiol Ment Health 2016;12:24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vardy J, Wong K, Yi Q‐l, et al. Assessing cognitive function in cancer patients. Support Care Cancer 2006;14(11):1111–8. [DOI] [PubMed] [Google Scholar]
- 20. Cheung YT, Lim SR, Shwe M, Tan YP, Chan A. Psychometric properties and measurement equivalence of the English and Chinese versions of the functional assessment of cancer therapy‐cognitive in Asian patients with breast cancer. Value Health 2013;16(6):1001–13. [DOI] [PubMed] [Google Scholar]
- 21. Cheung YT, Foo YL, Shwe M, et al. Minimal clinically important difference (MCID) for the functional assessment of cancer therapy: cognitive function (FACT‐Cog) in breast cancer patients. J Clin Epidemiol 2014;67(7):811–20. [DOI] [PubMed] [Google Scholar]
- 22. Erlanger DM, Kaushik T, Broshek D, Freeman J, Feldman D, Festa J. Development and validation of a web‐based screening tool for monitoring cognitive status. J Head Trauma Rehabil 2002;17(5):458–76. [DOI] [PubMed] [Google Scholar]
- 23. Food and Drug administration (FDA) . Bioanalytical Method Validation Guidance for Industry; 2018.
- 24. Murman DL. The impact of age on cognition. Semin Hear 2015;36(3):111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilson RS, Hebert LE, Scherr PA, Barnes LL, Mendes de Leon CF, Evans DA. Educational attainment and cognitive decline in old age. Neurology 2009;72(5):460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Neu D, Kajosch H, Peigneux P, Verbanck P, Linkowski P, Le Bon O. Cognitive impairment in fatigue and sleepiness associated conditions. Psychiatry Res 2011;189(1):128–34. [DOI] [PubMed] [Google Scholar]
- 27. Robinson OJ, Vytal K, Cornwell BR, Grillon C. The impact of anxiety upon cognition: perspectives from human threat of shock studies. Front Hum Neurosci 2013;7:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Szelenberger W, Niemcewicz S. Severity of insomnia correlates with cognitive impairment. Acta Neurobiol Exp 2000;60(3):373. [DOI] [PubMed] [Google Scholar]
- 29. Williams AM, Shah R, Shayne M, et al. Associations between inflammatory markers and cognitive function in breast cancer patients receiving chemotherapy. J Neuroimmunol 2018;314:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lokken KL, Ferraro FR. The relationship between menopausal status, phase of menstrual cycle, and replacement estrogen on cognition in healthy women without dementia. J Psychol 2006;140(6):533–47. [DOI] [PubMed] [Google Scholar]
- 31. Davis SR, Shah SM, McKenzie DP, Kulkarni J, Davison SL, Bell RJ. Dehydroepiandrosterone sulfate levels are associated with more favorable cognitive function in women. J Clin Endocrinol Metab 2008;93(3):801–8. [DOI] [PubMed] [Google Scholar]
- 32. Vega JN, Dumas J, Newhouse PA. Cognitive effects of chemotherapy and cancer‐related treatments in older adults. Am J Geriatr Psychiatry 2017;25(12):1415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Forti P, Maltoni B, Olivelli V, Pirazzoli GL, Ravaglia G, Zoli M. Serum dehydroepiandrosterone sulfate and adverse health outcomes in older men and women. Rejuvenation Res 2012;15(4):349–58. [DOI] [PubMed] [Google Scholar]
- 34. Lange M, Giffard B, Noal S, et al. Baseline cognitive functions among elderly patients with localised breast cancer. Eur J Cancer 2014;50(13):2181–9. [DOI] [PubMed] [Google Scholar]
- 35. Ravaglia G, Forti P, Maioli F, et al. The relationship of dehydroepiandrosterone sulfate (DHEAS) to endocrine‐metabolic parameters and functional status in the oldest‐old. Results from an Italian study on healthy free‐living over‐ninety‐year‐olds. J Clin Endocrinol Metab 1996;81(3):1173–8. [DOI] [PubMed] [Google Scholar]
- 36. Yamada S, Akishita M, Fukai S, et al. Effects of dehydroepiandrosterone supplementation on cognitive function and activities of daily living in older women with mild to moderate cognitive impairment. Geriatr Gerontol Int 2010;10(4):280–7. [DOI] [PubMed] [Google Scholar]
- 37. Hildreth KL, Gozansky WS, Jankowski CM, Grigsby J, Wolfe P, Kohrt WM. Association of serum dehydroepiandrosterone sulfate and cognition in older adults: sex steroid, inflammatory, and metabolic mechanisms. Neuropsychology 2013;27(3):356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Apple AC, Schroeder MP, Ryals AJ, et al. Hippocampal functional connectivity is related to self‐reported cognitive concerns in breast cancer patients undergoing adjuvant therapy. Neuroimage Clin 2018;20:110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dong Y, Zheng P. Dehydroepiandrosterone sulphate: action and mechanism in the brain. J Neuroendocrinol 2012;24(1):215–24. [DOI] [PubMed] [Google Scholar]
- 40. Starka L, Duskova M, Hill M. Dehydroepiandrosterone: a neuroactive steroid. J Steroid Biochem Mol Biol 2015;145:254–60. [DOI] [PubMed] [Google Scholar]
- 41. Hucklebridge F, Hussain T, Evans P, Clow A. The diurnal patterns of the adrenal steroids cortisol and dehydroepiandrosterone (DHEA) in relation to awakening. Psychoneuroendocrinology 2005;30(1):51–7. [DOI] [PubMed] [Google Scholar]
- 42. Vermeulen A, Verdonck L. Plasma androgen levels during the menstrual cycle. Am J Obstet Gynecol 1976;125(4):491–4. [DOI] [PubMed] [Google Scholar]
- 43. Chemaitilly W, Sklar CA. Endocrine complications in long‐term survivors of childhood cancers. Endocr Relat Cancer 2010;17(3):R141–59. [DOI] [PubMed] [Google Scholar]
- 44. Huppert FA, Van Niekerk JK, Herbert J. Dehydroepiandrosterone (DHEA) supplementation for cognition and well‐being. Cochrane Database Syst Rev 2000;(2):Cd000304. [DOI] [PubMed] [Google Scholar]
- 45. Rutkowski K, Sowa P, Rutkowska‐Talipska J, Kuryliszyn‐Moskal A, Rutkowski R. Dehydroepiandrosterone (DHEA): hypes and hopes. Drugs 2014;74(11):1195–207. [DOI] [PubMed] [Google Scholar]
- 46. Cheung YT, Tan EH, Chan A. An evaluation on the neuropsychological tests used in the assessment of postchemotherapy cognitive changes in breast cancer survivors. Support Care Cancer 2012;20(7):1361–75. [DOI] [PubMed] [Google Scholar]
- 47. Hutchinson AD, Hosking JR, Kichenadasse G, Mattiske JK, Wilson C. Objective and subjective cognitive impairment following chemotherapy for cancer: a systematic review. Cancer Treat Rev 2012;38(7):926–34. [DOI] [PubMed] [Google Scholar]
- 48. Pluchino N, Drakopoulos P, Bianchi‐Demicheli F, Wenger JM, Petignat P, Genazzani AR. Neurobiology of DHEA and effects on sexuality, mood and cognition. J Steroid Biochem Mol Biol 2015;145:273–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Development and validation for the methodology for quantitative determination of plasma DHEA(S) levels.
Table S2A. Association of DHEA(S) and clinically relevant factors with self‐perceived CRCI.
Table S2B. Association of DHEA(S) and clinically relevant factors with objective CRCI.
Figure S1. (A) Graph plot of global FACT‐Cog scores against time points for cognitively impaired patients. (B). Graph plot of global FACT‐Cog scores against time points for non–cognitively impaired patients.


