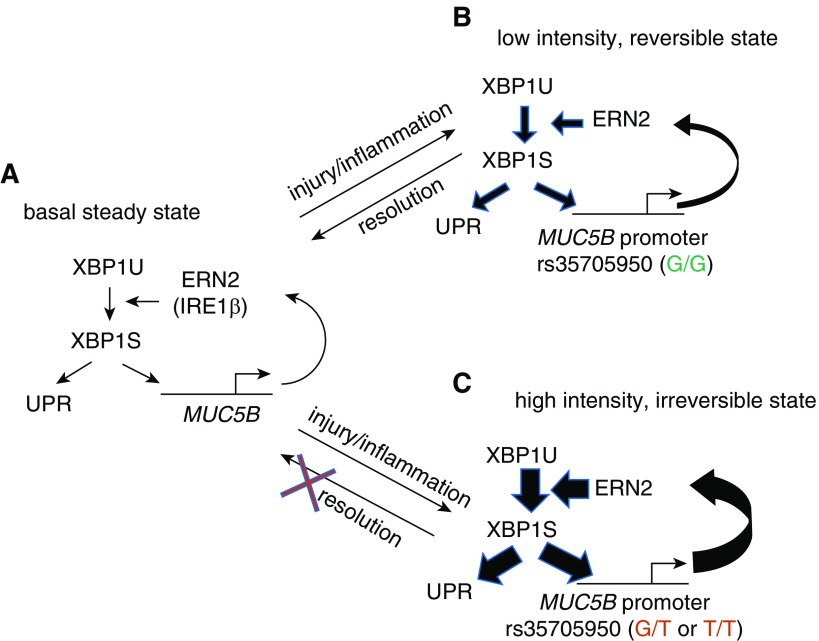
Figure 8.
A “bistable” model of ERN2/XBP1S-mediated regulation of MUC5B and its promoter variant in distal airway epithelia of idiopathic pulmonary fibrosis. (A) Basal MUC5B transcription produces MUC5B mRNAs that are translated into MUC5B protein at the endoplasmic reticulum (ER). The MUC5B protein synthetic load is sensed by ERN2 (and perhaps also by ERN1), which leads to splicing of XBP1U (unspliced XBP1) to XBP1S. XBP1S regulates basal level expression of MUC5B and unfolded protein response (UPR) genes. Note, each step reflects a positive regulatory interaction. (B) In a rs35705950 (G/G) distal airway mucin-secreting cell, inflammatory cytokines or other types of injury/stimuli stimulate MUC5B expression. An increased MUC5B mRNA load fuels increased MUC5B synthesis in ER, increased ERN2 activity, and XBP1S formation, and positive feedback by XBP1S on MUC5B transcriptional activity. The strength of this interaction is relatively weak, reflecting the “low-intensity state” of the XBP1S–MUC5B (G/G) promoter interaction. On removal of the stress-causing stimuli, the cell can revert to a low MUC5B basal secretion, low UPR activation state. (C) In a MUC5B rs35705950 (G/T or T/T) promoter distal airway mucus-secreting cell, stress signaling triggers overloaded MUC5B mRNA and protein synthesis, which in turn leads to ERN2 hyperactivation and increased XBP1S formation. Note, the regulatory interaction in the MUC5B promoter between XBP1S and rs35705950 locus is amplified by the presence of G/T or T/T at that site. This positive feedback reflects a “high-intensity state” of interaction, which produces a high-intensity signal response that imposes significant ER stress and may be irreversible. This state is sufficient to trigger an abnormal response to impair innate host defense and contribute to cell senescence. A second hit (e.g., oxidative stress) may signal these already “high-intensity” cells to accentuate ER stress–induced injury further, resulting in a profibrotic microenvironment that promotes fibroblast proliferation and myofibroblast differentiation (not shown in the illustration).