Figure 1.
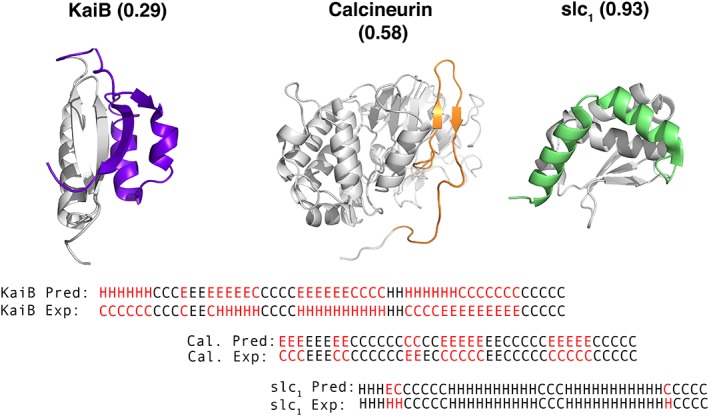
Secondary structure predictions of FSRs span a wide range of accuracies. Q 3 scores range from 0.29 for the inactive tetrameric form of KaiB (purple, pdb ID: 2QKE_A) to 0.58 for the cis conformation of calcineurin (orange, pdb ID:5C1V_B), and 0.93 for the monomeric form of archaeal selecase slc1 (green, pdb ID: 4QHF_A). Alignments of predicted and experimental secondary structures are shown below protein structures; black letters are consistent; red are inconsistent. Secondary structure predictions were made using JPred4.
