Figure 4.
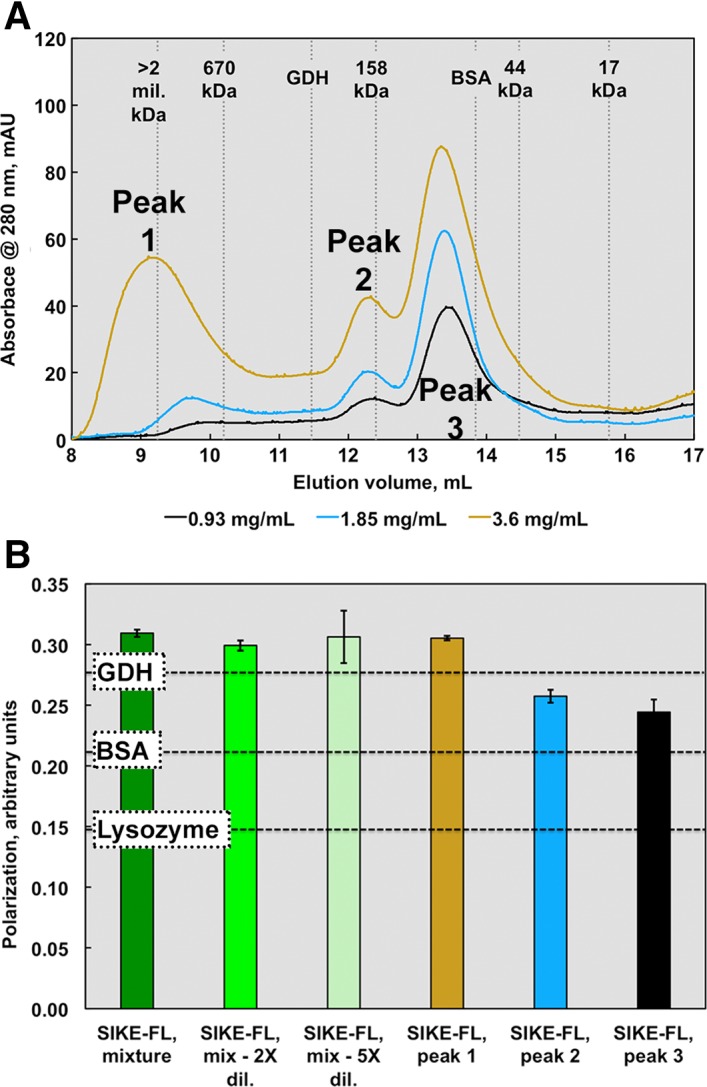
SEC and fluorescence polarization data suggest an elongated or multimeric SIKE structure. SIKE (0.9–3.6 mg/mL or 36–150 μM, colored cyan to dark blue), separated on an ENrich SEC 650 column, showed three distinct species. Peak 1 was assumed an aggregated species with MW corresponding to >1 mil Da. If SIKE behaves as a spherical globular protein in solution similar to the proteins used to derive the standard curve, Peak 2 would correspond to 182 kDa species (7mer based upon a SIKE monomer MW of 25.9 kDa) and Peak 3 a trimer of 86 kDa. The area of Peaks 1 and 2 increased with SIKE concentration. When Peak 1 was subjected to a second SEC separation, the protein retained its initial elution pattern, suggesting aggregation was not reversible (data not shown). (B) Protein fluorescence polarization of SIKE (EX 295 nm; EM 340 nm) at 0.1 mg/mL suggested a species larger than the protein standard GDH (MW 334 kDa) with dilution to 0.05 or 0.02 mg/mL having no effect on overall polarization of sample, consistent with species in an irreversible, aggregated state. Polarization measurements of individual peaks from the 2.5 mg/mL SEC experiment revealed that polarization values from Peaks 2 and 3 were bound by BSA and GDH values, consistent with the SEC data. Both experiments indicate that, in solution, SIKE does not behave as a globular, monomeric 25.9 kDa protein.
