Abstract
The past 100 yr have seen dramatic philosophical shifts in our approach to controlling or managing pest species. The introduction of integrated pest management in the 1970s resulted in the incorporation of biological and behavioral approaches to preserve ecosystems and reduce reliance on synthetic chemical pesticides. Increased understanding of the local ecosystem, including its structure and the biology of its species, can improve efficacy of integrated pest management strategies. Pest management strategies incorporating insect learning paradigms to control insect pests or to use insects to control other pests can mediate risk to nontarget insects, including pollinators. Although our understanding of insect learning is in its early stages, efforts to integrate insect learning into pest management strategies have been promising. Due to considerable differences in cognitive abilities among insect species, a case-by-case assessment is needed for each potential application of insect learning within a pest management strategy.
Keywords: conditioning, aversive, associative, learned behavior, memory
Learning and memory are ubiquitous among animals, including insects (Capaldi et al. 1999, Brown and Laland 2003, Griffin 2004, Hoppitt and Laland 2008). Most research on insect behavior and learning have focused on aspects that might improve the productivity of beneficial insects or help mediate pest insects (Capaldi et al. 2000; Kawaguchi et al. 2006; Vinauger et al. 2011a, b; Giunti et al. 2015; I’ Anson Price et al.2019). Numerous definitions of learning have been proposed, many of which reference a behavioral change in response to experience often involving experience-induced changes in neurophysiology (van Alpern and Vet 1986, Barron 2001, Dukas 2008). Learning requires memory, which can be described as the persistence of learning or the retention and retrieval of learned information (Menzel and Müller 1996, Okano et al. 2000, McCall and Kelly 2002, Frost et al. 2012). Factors to consider in identifying learned behavior include repeatability of behavioral changes, gradual changes with continued experience, and decay in behavioral changes in the absence of continued experience or in the presence of different experience, demonstrating that learning is a life-long process rather than an outcome (Papaj and Prokopy 1989). Here we provide an overview of the history of insect learning and potential applications.
Insect Learning and Memory
Complex behaviors such as food- and mate-finding are adaptive responses involving the performance of multiple integrated different behaviors and can rely on a suite of innate (inherited) preferences and preferences acquired through learning in response to multimodal environmental, physiological, or social cues (Papaj and Prokopy 1989; Turlings et al. 1989; Vet et al. 1998, 1990; Segura et al. 2007; Jones et al. 2015; Enjin et al. 2016). The ability of a species to learn can be closely tied to its phenotypic plasticity (e.g., behavior), which itself can be a blend of adaptability to novel experiences and complex innate programming (Kaiser et al. 1989, Sachse et al. 2007, Dukas 2008, Pfennig et al. 2010). Behavioral plasticity can be described as the ability of an individual to adapt to new or changing information, assess its relevance, and prioritize information according to relative reliability of available information (Kaiser et al. 1989, Sachse et al. 2007, Dukas 2008, Pfennig et al. 2010). In general terms, species that inhabit a more diverse or changeable habitat, or that use a wider variety of hosts, may have greater reliance on learned sensory cues associated with suitable hosts. Learning is profitable where habitats are variable among generations or change rapidly or unpredictably within an insect’s lifetime, as evidenced by foraging bumblebees (Bombus spp. Latreille [Hymenoptera: Apidae]) observing conspecifics to identify ephemeral floral resources (Stephens 1993, Dunlap et al 2016). Conversely, a uniform and unchanging habitat could limit the importance of learning in favor of evolved innate preferences. Species which rely on greater diversity of hosts (generalists) could benefit more from learning cues associated with host finding whereas specialist species could more easily rely on innate preferences (Segura et al. 2007).
Most studies of insect learning have focused on responses to olfactory and visual stimuli (Thorpe and Jones 1937, Wäckers and Lewis 1994, McCall and Eaton 2001, Segura et al. 2007, Busto et al. 2010, Benelli and Canale 2012, Liu and Sakuma 2013, Vinauger et al. 2014). However, insects can learn using a variety of sensory cues, including olfactory, visual (color, contrast, pattern, shape, and size), gustatory/taste, tactile/auditory, temperature, and spatial/temporal signals (Table 1). In some cases, learning paradigms can be extremely complex. Dung beetles (Scarabaeus (Kheper) lamarcki MacLeay [Coleoptera: Scarabaeidae]) learn to orient using a fixed-time map of celestial cues as a navigational guide (el Jundi et al. 2016). Leaf-cutter ants (Acromyrmex ambiguous Emery [Hymenoptera: Formicidae]) learn to avoid leaves treated with fungicide, which is undetectable to the ants, based on growth patterns in their symbiotic fungal gardens (Saverschek et al. 2010, Saverschek and Roces 2011). Honeybees (Apis mellifera Linnaeus [Hymenoptera: Apidae]) are highly adaptable, continually incorporating new experiences, including weather conditions, flower availability, and colony conditions, and can learn conceptual relationships, including more/less, above/below, left/right, and same/different (Capaldi et al. 2000, Avarguès-Weber and Giurfa 2013).
Table 1.
Although olfactory cues are most commonly used in studies of learning in insects, a variety of sensory cues have been demonstrated as effective stimuli for complex associative learning behaviors.
| Order: Family | Species | Sensory System | Stimuli | Source |
|---|---|---|---|---|
| Diptera: Drosophilidae | Drosophila melanogaster Meigen | Olfactory / Tactile | Food odors and mechanical disturbance | Saumweber et al. 2014 |
| Hymenoptera: Apidae | Apis mellifera Linnaeus (honeybees) | Tactile / Acoustic | airborne sound | Kirchner et al. 1991 |
| Visual | complex natural scenes | Dyer et al. 2008 | ||
| Visual | Colors and shapes | Buatois et al. 2017 | ||
|
Bombus terrestris Linnaeus (bumblebees) |
Gustatory / Taste | cues in nectar | Molet et al. 2009 | |
| Visual | flower color | Leadbeater and Chittka 2007 | ||
| Visual | flower color | Dunlap et al. 2016 | ||
| Visual / Olfactory | flower color and blue/ yellow | Smith and Raine 2014 | ||
| Hymenoptera: Formicidae |
Atta vollenweideri Forel (leaf-cutter ants) |
Temperature | thermal radiation | Kleineidam et al. 2007 |
|
Cataglyphis noda Brullé (desert ants) |
Tactile / Acoustic | vibration and magnetic fields | Buehlmann et al. 2012 | |
|
Myrmica rubra Linnaeus (red ants) |
Spatial / Temporal | routes to disposal sites | Diez et al. 2011 |
Studies into insect learning have been focused primarily on honeybees and other pollinators (foraging behavior and sublethal effects of pesticides and toxins), predatory insects and parasitoids (host-finding behavior and potential for improved efficacy in pest management strategies), Drosophila spp. Fabricius (Diptera: Drosophilidae) (as a model system for understanding insect development, evolution, and genetics), and phytophagous insects (host-selection and host-finding behavior; Du et al. 1997, Cunningham et al. 2001, Schwaerzel et al. 2003, Fiala 2007, Leadbeater and Chittka 2009, Busto et al. 2010, Giunti et al. 2015, Charbonneau et al. 2016, Dunlap et al. 2016, Smolla et al. 2016). Learning and memory can be assessed via changes in insect behavior (choice tests), involuntary responses (proboscis extension reflex), or physiology (calcium imaging; electromyography and silicon multichannel electron arrays; Faber et al. 1999, Daly et al. 2004, Farina et al. 2005, Frost et al. 2012). Measuring or quantifying learning and memory can be challenging because it can be difficult to determine whether behavioral change is due to learning or other factors, such as change in motivation, fatigue, physiological changes, or injury (Barron et al. 2015). In addition, differences in conditioning methods, experimental design, and measurement techniques can significantly influence results and their interpretation (Frost et al. 2012).
Types of Insect Learning
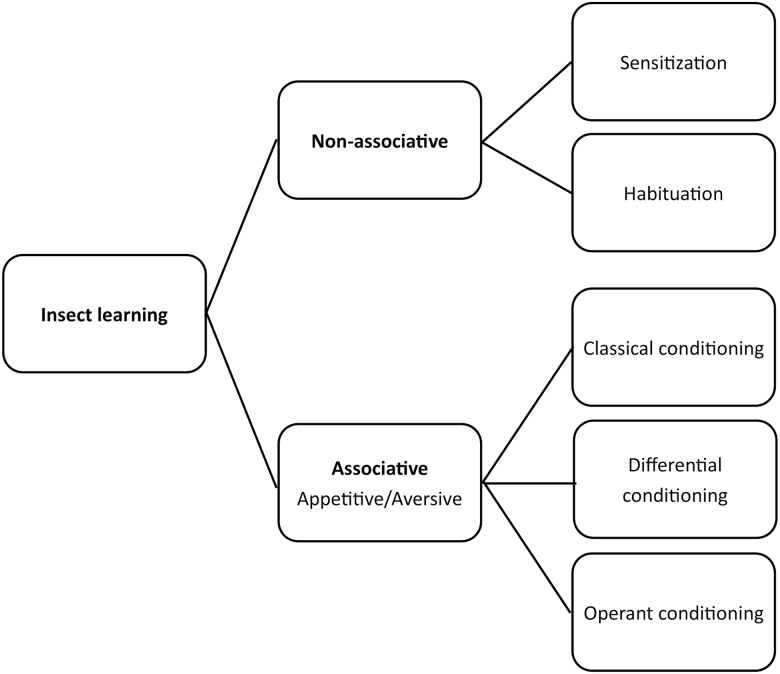
Insect learning can occur through several different processes (Fig. 1). Non-associative learning is the simplest form, comprised of becoming more (sensitization) or less (habituation) sensitive to a sensory cue through exposure. Responses can be induced by short-term exposure at critical points in development (e.g., during or shortly following emergence). Through repeated or prolonged exposure, sensitization may form the basis for sensory memory (Stopfer and Laurent 1999, Galizia and Sachse 2010). Sensitization and habituation of olfactory cues occur in the antennal lobes or mushroom bodies (Faber et al. 1999, Farooqui et al. 2003, Daly et al. 2004, Larkin et al. 2010).
Fig. 1.
Forms of insect learning. Even a single exposure to a stimulus without an associated reward or punishment can induce a familiarization effect. These non-associative conditioning events can enhance sensitivity (sensitization) resulting in increased response to innately attractive stimuli or in reduce sensitivity (habituation) resulting in decreased avoidance of innately repellent stimuli. Associative conditioning occurs when exposure to a stimulus precedes or occurs concurrently with a positive (appetitive conditioning) or negative (aversive conditioning) reward experience. Classical (Pavlovian) conditioning occurs when the induced behavioral response is involuntary or reflexive (proboscis extension in honeybees, salivation in dogs). In contrast, operant conditioning occurs when the induced response is a modifiable behavior. Differential conditioning combines any of the preceding conditioning paradigms or involves differing reward values for differing stimuli.
Associative learning refers to conditioning the individual to associate a stimulus cue to either a positive reward (appetitive learning) or a negative punishment (aversive learning). Appetitive and, to a lesser extent, aversive conditioning have been demonstrated in most insect orders, with extensive studies involving Hymenoptera, Diptera, Blattodea, Lepidoptera, Coleoptera, and Hemiptera (Ebeling et al. 1966; Brodie Jr and Formanowicz Jr 1981; Meller and Davis 1996; Schwaerzel et al. 2003; Jørgensen et al. 2007; Saverschek and Roces 2011; Vinauger et al. 2011a, b; Buatois et al. 2017).
Associative learning can take the form of classical (Pavlovian) conditioning (Fig. 1), in which the stimulus induces a learned reflexive response (i.e., proboscis extension), or operant conditioning, where the stimulus induces a learned modifiable behavioral response (Garren et al. 2013). The strength of the response in operant conditioning varies with the value of the reward. Differential conditioning combines associative and non-associative learning paradigms (Faber et al. 1999). Associative memories and memories that involve a combination of olfaction, visual, spatial, and tactile cues, are retained in the mushroom body (de Belle and Heisenberg 1994, Armstrong et al. 1998, Hammer and Menzel 1998, Strausfeld et al. 1998, Schwaerzel et al. 2003, Farooqui et al. 2003). Prolonged or repeated experiences can result in permanent changes to behavior and physiology, including antennal lobes and mushroom bodies (Thorpe 1938, Hershberger and Smith 1967, Devaud et al. 2001, Farris et al. 2001, Sachse et al. 2007).
Insect Life Stage and Learning
The influence of learning and memory retention across life stages in insects, particularly holometabolous insects, has been hotly debated over the past 100 yr (Hopkins 1916, 1917; Craighead 1921, 1923; Thorpe and Jones 1937; Thorpe 1939; Thompson 1988; Jaenike and Holt 1991; Tully et al. 1994; Barron and Corbet 1999; Barron 2001; Gandolfi et al. 2003a; Schroll et al. 2006; Blackiston et al. 2008; Bonduriansky and Day 2009; Davis 2008; Anderson et al. 2013; Aso et al. 2014; Ernst et al. 2015). Although metamorphosis dramatically alters insect physiology, including extensive degeneration, reorganization, and regrowth of antennal lobe and mushroom body structures, select underlying neuronal cells and portions of the mushroom body can be traced from larval to adult stages (Armstrong et al. 1998, Blackiston et al. 2008, Aso et al. 2014). Although mushroom body structures and degree of developmental continuity differ among insect taxa, potential may exist for some conservation of memory (Blackiston et al. 2008).
Larval insects are capable of robust but simplified odor perception and odor learning (Scherer et al. 2003, Hendel et al. 2005, Gerber and Stocker 2007). Among some taxa, natal experience is thought to influence adult behavior through imprinting (sensitization or habituation) or associative learning (Tully et al. 1994, Blackiston et al. 2008, Davis 2008). Results vary among studies and learning can sometimes be partly attributed to experience during or immediately after adult emergence, such as sensory cues located on or near the pupal case, rather than at larval stages (Thorpe and Jones 1937; Thorpe 1939; Barron and Corbet 1999, 2000; Gandolfi et al. 2003a; Anderson et al. 2013). The relationship between larval experience and adult host preference can also be due to induced gene expression or a genetically distinct subset of the population (Thompson 1988, Jaenike and Holt 1991, Schroll et al. 2006, Bonduriansky and Day 2009). Residual host or environmental odors experienced during adult emergence can induce a chemical legacy association to find new hosts (Corbet 1985, Veltman and Corbet 1991, Rietdorf and Steidle 2002, Gandolfi et al. 2003a, Blackiston et al. 2008). During postemergence reconnaissance flights, adult parasitic wasps (Hymenoptera) learn olfactory and visual cues of prey’s host plants associated with successful ovipositing to identify potential future oviposition sites (Tumlinson et al. 1993).
Recent advances in DNA sequencing technology and identification of a functional DNA methylation system in A. mellifera have raised questions about the role of epigenetics in host-switching (Ernst et al. 2015). Many insect species express different phenotypes due to seasonal or regional differences in available diet or environmental conditions during development that could explain differences in sensitivity to sensory stimuli and the resulting host preference hierarchies (Pfennig et al. 2010, Ernst et al. 2015, Kijimoto and Moczek 2016).
Among adult insects, the reliability of sensory cues as predictors for relevant aspects of environment is key to determining the value of learned cues (Dunlap et al. 2016). Negative courtship experiences can cause male Drosophila melanogaster Meigen (Diptera: Drosophilidae) to have increased sensitivity to male pheromone on female flies and improve discrimination between mated and virgin females (Keleman et al. 2012).
Memory is either reinforced through repeated experience or decays where memory of experience-associated odors lacks benefits (Grossmann 1973, Vet and Dicke 1992, Kaiser and De Jong 1993, McCall et al. 1993, Menzel and Müller 1996, Busto et al. 2010, I’Anson Price et al. 2019). Depending upon the species and habitat variability, experience can result in host preferences that vary in persistence from a few minutes to many days but tends to persist longer with subsequent repeated experience (Kaiser et al. 1989, Bjorksten and Hoffmann 1998, Blackiston et al. 2008, Collett 2008, Vinauger et al. 2014). Short-term, intermediate, and long-term memory phases have been identified in honeybees, mosquitoes (Diptera: Culicidae), and Drosophila (Meller and Davis 1996, Vinauger et al. 2014). Short-term (persisting minutes to hours) and intermediate memory (persisting hours to days) can be formed following a single exposure or conditioning experience (Meller and Davis 1996, Busto et al. 2010, Vinauger et al. 2014). Long-term memory (persisting days or longer) can require repeated reinforcement experiences (Grossmann 1973, Kaiser and De Jong 1993, Meller and Davis 1996, Busto et al. 2010, Saverschek et al. 2010). Simultaneous exposure to multiple conditioning experiences can force insects to prioritize, inhibiting learning of cues associated with one experience in favor of the other (Rains et al. 2008, Christiansen and Schausberger 2017). As experience grows, learning can serve to modify or reinforce learned or innate preferences, often by introducing local adaptations (Kaiser et al. 1989, Galef Jr 1995, McCall and Kelly 2002).
Within a taxonomic class of several million species, it is unlikely that a ‘one size fits all’ hypothesis would apply to all cases. Changes in host preferences are likely attributable to multiple factors, including genetics, selection, and conditioning (Barron 2001). In limited circumstances, larval conditioning may play a role in adult behavior but is generally superseded by adult experiences and conditioning (Barron 2001, Blackiston et al. 2008). Differences of opinion about timing, permanence, and basis of insect learning are likely to persist for many years to come. However, there is growing acknowledgment of the existence of a variety of insect learning models and the importance of learning for adaptive behavior by both pest and beneficial insect species.
Social Learning
Foraging, particularly by naïve individuals, or to locate novel food sources, is time-consuming and risky (Chittka and Leadbeater 2005, Dunlap et al. 2016, Smolla et al. 2016). Learning from conspecifics is well documented among insects, particularly social insects such as honeybees (A. mellifera), bumblebees (Bombus spp.), and ants Linnaeus (Hymenoptera: Formicidae) (Chittka and Leadbeater 2005, Coolen et al. 2005, Leadbeater and Chittka 2007). Learning via observation promotes the rapid spread of novel information or behaviors, such as watching conspecifics trying novel hosts or food sources (Leadbeater and Chittka 2007, Sarin and Dukas 2009, Giurfa 2012, Durisko and Dukas 2013, Dunlap et al. 2016).
Social learning allows individuals to reduce risks of mistakes or negative experiences that could occur through trial-and-error, provided the information source is considered reliable (Jones et al. 2015, Dunlap et al. 2016, Smolla et al. 2016, I’Anson Price et al. 2019). Naïve wasps, bees, and ants learn to recognize physical and chemical cues from novel food sources by observing nest-mates and are attracted by foraging conspecifics, but more experienced individuals will avoid conspecifics at food sources, preferring less competitive feeding sites (Slaa et al. 2003, Kawaguchi et al. 2006, Leadbeater and Chittka 2007). Social cues can include observing conspecifics at feeding sites/flowers, odor ‘footprints’ left at feeding sites, or floral odors carried into the nest by nest-mates (Molet et al. 2009). Eusocial insects, such as honeybees, bumblebees, and ants, have been observed engaging in recruitment behavior akin to mentoring, such as honey bee waggle dancing and ant trail marking (Chittka and Leadbeater 2005, Franks and Richardson 2006, Smolla et al. 2016). Analyses of recruitment relative to food patch distribution patterns suggest that this behavior is learned rather than innate (Heinrich 1979, Leadbeater and Chittka 2009). When recruitment messages are unreliable, foragers quickly learn to ignore social cues in favor of increased scouting activity (Dunlap et al. 2016, I’Anson Price et al. 2019). Nonsocial insects, including crickets Laicharting (Orthoptera: Gryllidae), damselflies Sélys (Odonata: Zygoptera), and flies Linnaeus (Diptera), learn to associate chemical cues of wounded conspecifics to avoid predators and learn about resources or avoid predators by observing heterospecifics (Wisenden et al. 1997, Chittka and Leadbeater 2005, Coolen et al. 2005, Leadbeater and Chittka 2007, Roberts 2012).
Insect Learning and Pest Management Strategies
Numerous insect species are classified as pests primarily due to widespread damage to agricultural and forestry plant products and the spread of disease to human and animal hosts. Integrated pest management strategies have been developed in response to many of these threats to food safety and health. However, pesticide resistance and changing efficacy of pest management strategies continue to be a problem (Kogan 1998, Bass et al. 2015). The idea that biological and chemical pest controls could supplement each other was first discussed in 1939 and reintroduced in 1954 (Hoskins et al. 1939, Smith and Allen 1954). However, the integration of chemical and biological pest management strategies did not become widespread until the 1970s, spurred by the growing awareness of consequences of over-reliance of pesticides (Carson 1962).
Researchers have begun exploring the potential to integrate insect learning to improve the efficacy of pest management practices, primarily in the areas of agriculture and forestry, disease-vector control (medical and veterinary), and urban pest management (Dukas 2008, Menzel 2012, Leadbeater and Dawson 2017). Many insect species integrate multimodal sensory cues, including olfactory, visual, tactile, acoustic, and spatial stimuli, to identify hosts and discount nonhosts (Smith and Allen 1954, Campbell and Borden 2009). Conditioning paradigms that combine multimodal stimuli can reinforce learned preferences in parasitoids or predatory insects (Table 2). Exposure to pesticides and other pest management efforts may result in unintended avoidance conditioning experiences for pest insects. Understanding these processes could inform our efforts to limit the efficacy of these learned avoidance behaviors (Table 3).
Table 2.
A variety of studies have been completing investigating insect learning by Hymenopteran parasitoids of importance to pest management strategies. Researchers have sought to improve the efficacy of pest management efforts against a diverse range of pest insects using a variety of stimuli and conditioning paradigms.
| Time of Conditioning | Parasitoid | Pest Species | Stimuli | Reference |
|---|---|---|---|---|
| Prior to / during emergence | Hyssopus pallidus Askew (Hymenoptera: Eulophidae) | Cydia pomonella Linnaeus (Lepidoptera: Tortricidae) | kairomones in larvae and frass | Gandolfi et al. 2003a, b |
| During / postemergence | Nemeritis canescens Gravenhorst (Hymenoptera: Ichneumonidae) | Ephestia kilhniella Zeller (Lepidoptera: Pyralidae) and E. elutella Hübner (Lepidoptera: Pyralidae) | host kairomones | Thorpe and Jones 1937 |
| Postemergence | Dinarmus basalis Rondani (Hymenoptera: Pteromalidae) | Zabrotes subfasciatus Boheman (Coleoptera: Bruchidae) and Acanthoscelides obtectus Say (Coleoptera: Bruchidae) | host kairomones | Caubet and Jaisson 1991 |
| Trichogramma nr. Brassicae Bezdenko (Hymenoptera: Trichogrammatidae) | Sitotroga cerealella Olivier (Lepidoptera: Gelechiidae) and Helicoverpa punctigera Wallengren (Lepidoptera: Noctuidae) | host kairomones | Bjorksten and Hoffmann 1998 | |
| Microplitis croceipes Cresson (Hymenoptera: Braconidae) | Helicoverpa zea Boddie (Lepidoptera: Noctuidae) | host-plant semiochemicals | Drost et al. 1988 | |
| Cotesia marginiventris Cresson (Hymenoptera: Braconidae) | Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) and Trichoplusia ni Hübner (Lepidoptera: Noctuidae) | host-plant semiochemicals and kairomones in frass | Lewis et al. 1991 | |
| Cotesia marginiventris and Microplitis croceipes | H. zea | host-plant semiochemicals and kairomones in frass | Turlings et al. 1989, 1993 | |
| Microplitis demoliter Wilkinson (Hymenoptera: Braconidae) | H. zea | host-plant semiochemicals in frass | Hérard et al. 1988 | |
| Aphytis melinus DeBach (Hymenoptera: Aphelinidae) | Aonidiella aurantia Maskell (Hemiptera: Diaspididae) | kairomone in host cuticle | Hare et al. 1997 | |
| Cotesia marginiventris | Spodoptera frugiperda | kairomone in host frass | Loke and Ashley 1984 | |
| Microplitis croceipes, Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae), and T. achaeae Nagaraja (Hymenoptera: Trichogrammatidae), and Nagarkatti (Hymenoptera: Trichogrammatidae) | H. zea | kairomone in host frass | Gross et al. 1975 | |
| Pre-adult and during oviposition | Trichogramma nr ivelae Pang and Chen (Hymenoptera: Trichogrammatidae) | H. punctigera, Papilo Aegeus Donovan (Lepidoptera: Papilionidae), and Hypolimnus bolina Linnaeus (Lepidoptera: Nymphalidae) | host kairomones | Bjorksten and Hoffmann 1995 |
| During oviposition | Psyttalia concolor Szépligeti (Hymenoptera: Braconidae) | Ceratitis capitate Wiedemann (Diptera: Tephritidae) | color | Benelli and Canale 2012 |
| Leptopilina heterotoma Thomson (Hymenoptera: Eucoilidae) | Drosophila simulans Sturtevant (Diptera: Drosophilidae) | host-infested fruit odors | Vet et al. 1990 | |
| Cotesia glomerate Linnaeus (Hymenoptera: Braconidae) | Pieris brassicae Linnaeus (Lepidoptera: Pieridae) and Spodoptera littoralis Boisduval (Lepidoptera, Noctuidae) | host-infested plant odors | Desurmont et al. 2018 | |
| Lysiphlebus fabarum Marshall (Hymenoptera: Braconidae) | Aphis fabae Scopoli (Hemiptera: Aphididae) | social cues | Rasekh et al. 2010 | |
| During repeated ovipositioning | Trichogramma achaeae | H. zea | kairomone in host cuticle | Lewis et al. 1975 |
| Multi-generational | Trichogramma maidis Pintureau and Voegele (Hymenoptera, Trichogrammatidae) | Ostrinia nubilalis Hübner (Lepidoptera: Crambidae) and Anagasta kuehniellu Zeller (Lepidoptera: Pyralidae) | host kairomones | Kaiser et al. 1989 |
Table 3.
Direct application of insect learning paradigms on integrated pest management programs.
| Pest category | Target insect | Type of conditioning | IPM practice | Reference |
|---|---|---|---|---|
| Herbivores | Acheta domesticus Linnaeus (Orthoptera: Gryllidae) | aversive | necromones to condition against food odors | Shephard et al. 2018 |
| Helicoverpa armigera Hübner (Lepidoptera: Noctuidae) | associative | integrating host abundance into plant volatile mixture in lures | Cunningham et al. 1999 | |
| generalist herbivores | associative | integrating host abundance into plant volatile mixture in lures | Shanower and Romeis 1999 | |
| aversive | toxic / bitter / distasteful compounds as deterrents | Bernays 1993 | ||
| aversive | nutrient deficiencies as deterrents | Bernays 1993 | ||
| aversive | intercropping | Verkerk et al. 1998 | ||
| disrupt associative | intercropping | Verkerk et al. 1998 | ||
| Disease vectors | Aedes aegypti Linnaeus (Diptera: Culicidae) | aversive | olfactory cues as blood-feeding deterrent | Menda et al. 2013 |
| Anopheles stephensi Liston (Diptera: Culicidae) | aversive | olfactory cues as ovipositioning deterrent | Reisen 1975 | |
| Rhodnius prolixus Stål (Hemiptera: Reduviidae) | aversive | olfactory cues as blood-feeding deterrent | Vinauger et al. 2011b |
Agriculture and Forestry
The origins of our understanding of insect learning were in agriculture and forestry, in the works of Hopkins, Craighead, and Walsh, and this remains the most widely studied venue (Walsh 1864; Hopkins 1916, 1917; Craighead 1921, 1923). Much of the work in these areas has focused on the use of polyphagous parasitoid wasps and predatory species to control pest insects (Table 2). Strategies involving the mass release of Hymenopteran parasites could be made more effective by conditioning parasitoids to combination of olfactory, visual, and/or tactile/acoustic stimuli associated with finding hosts, food, or mates (Giunti et al. 2015). By tying an appropriate color cue to first ovipositioning experience of naïve female parasitoids, we can condition mass-released parasitoids to seek out hosts in the fruit of that color (Benelli and Canale 2012). Timing of experience during a parasitoid’s life history can greatly influence the efficacy of learning and memory retention and will vary among species (Browne and Withers 2002, Decker et al. 2007, Giunti et al. 2015). Although some host odors may be learned by larvae, most odors, including plant-host complex odors, are learned during or after adult emergence (Caubet and Jaisson 1991, Weiss et al. 2004, Takemoto et al. 2009, Rasekh et al. 2010). Therefore, both the conditioning stimuli and timing of learning experiences are important for pest management efficacy.
Disease-Vector Control
Although insects are a primary vector of many important diseases, implications of and potential applications for insect learning have received less attention in areas relating to disease control. However, there have been a few notable exceptions including research into insecticide avoidance behavior of pyrethroid-resistant Anopheles gambiae Giles (Diptera: Culicidae) in areas where insecticide-treated nets are in common use for malaria prevention (N’Guessan et al. 2007). Pyrethroid resistance combined with habituation to pesticide odors is rapidly reducing the efficacy of protective netting in malaria-prone areas of Africa. Site fidelity has been documented for feeding and oviposition sites in Anopheles spp. and Culex spp. Linnaeus (Diptera: Culicidae), suggesting that female mosquitoes have learned to recognize specific locations as suitable (McCall and Kelly 2002). Aversive conditioning has also been documented in ovipositioning site preferences of Anopheles stephensi Liston (Diptera: Culicidae) (Reisen 1975). Studies of Rhodnius prolixus Stål (Hemiptera: Reduviidae) and Aedes aegypti Linnaeus in Hasselquist (Diptera: Culicidae) behavior demonstrate that blood-feeding insects are capable of learning associations between olfactory cues and both positive and negative reinforcement (Vinauger et al. 2011a, b; Menda et al. 2013). It is likely that repeated experience would reinforce the association and prolong the avoidance behavior. Exposure to DEET during a simulated blood-feeding experience can reduce the innate aversive response of A. aegypti to DEET, suggesting that habituation can reduce pest control chemical efficacy (Vinauger et al. 2014). A single odor can act as either an attractant or repellent dependent upon the prior experience of Culex quinquefasciatus Say (Diptera: Culicidae), demonstrating that experience can modify and even nullify innate preferences for specific olfactory cues (McCall and Eaton 2001). The potential influence of positive and negative experiences on insect behavior must be considered in developing pest management strategies. Because many of the insects that are disease vectors have some capacity for learning, strategies that employ aversive conditioning in relation to human-associated olfactory or visual cues could improve the efficacy of pest management efforts.
Urban Pest Management
Insect learning has received less attention in the field of urban pest management. Much of the research has focused on the behavior of cockroaches. Early experiments demonstrated that a combination of associative learning and habituation to light helps German cockroaches (Blattella germanica Linnaeus [Blattodea: Ectobiidae]) avoid insecticide-treated areas (Ebeling et al. 1966). Cockroaches can become habituated to mildly repellent contact insecticides, improving their efficacy in the long-term (Ebeling et al. 1966). Experience can induce attraction to an innately neutral odor and inhibition to an innately attractive odor, including aversive conditioning from sublethal exposure to bait-insecticides (Kells et al. 2005, Liu and Sakuma 2013). There is also growing evidence of social learning in cockroaches (Lihoreau and Rivault 2011). The reluctance to incorporate learning paradigms into urban pest management may be due in large part to widespread fear of insects among the general population (Kellert 1993).
Implications of Learning for Pest Management
Intercropping and Physical Barriers
Large single crop sites, indicative of modern industrial-style monoculture farming, are ideal venues for sensitization and associative learning by phytophagous insects. Insects have ample opportunity for repeated stimulus-positive reward experiences to reinforce learning. Learning can lead to restricted host use or limited host preference (host constancy), especially where one host species is predominant. An individual polyphagous insect may learn host constancy to forage more efficiently (Cunningham et al. 1999). In contrast, intercropping (use of a variety of plants within a crop area) has been used as an effective pest management strategy for centuries (Horwith 1985, Knörzer et al. 2009). Application of intercropping practices varies significantly, as does its relative efficacy in limiting insect learning (Verkerk et al. 1998). A variety of intercropping strategies are currently in use, including alternating plants within a row, alternating rows of plants, use of multiple varieties or species of plants in a single crop area, intercropping throughout the entire crop area, or creating a border area of intercropped plants. Additionally, many intercropping programs use one or more plant species or varieties with properties that are repellent to pest species or attractive to predatory species. Intercropping can disrupt appetitive learning processes in insects by introducing unrewarded or negative experiences that limit the reliability of stimulus information (Verkerk et al. 1998, Finch and Collier 2000).
Physical barriers, including greenhouses, growing tunnels, and pesticide-treated barrier nets can be effective to control or reduce the inflow of pest insects. However, hymenopteran parasitoids and pollinators have demonstrated a strong aptitude for aversive learning and can associate olfactory stimuli and negative consequences of pesticide contact, which can effectively limit the movement of beneficial insects (Vergoz et al. 2007, Carcaud et al. 2009, Martin et al. 2014).
Careful timing of repellent semiochemicals and pesticides during critical early stages may be sufficient for newly emerging insects to develop aversive conditioning to crop plants. Insects excluded from crop areas via repellents or physical barriers during early adult stages may instead become sensitized to olfactory and visual stimuli associated with non-crop plant hosts and through repeated feeding or ovipositioning, become conditioned to seek out non-crop hosts (Cunningham et al. 1998, 1999). This would require considerable understanding of pest insect life history and an effective early monitoring program to ensure pest management efficacy. In the case of multivoltine pest species, growers would need to reapply repellents or exclusion mechanisms with each successive generation to ensure efficacy.
Biological Control
A variety of parasitoid and predator insect control programs for agriculturally important pest insects are currently in use (Table 2). Most commonly, generalist parasitoid hymenopterans are reared under controlled conditions for mass-release in crop areas. For example, Aphytis melinus DeBach (Hymenoptera: Aphelinidae) are reared on oleander scale insects, which are usually reared on squash, and released commercially as a control agent for the citrus pest, California red scale (Aonidiella aurantii Maskell [Hemiptera: Diaspididae]). A single exposure of A. aurantii kairomones to newly emerged A. melinus, is sufficient to increase host preference for A. aurantii and improve efficacy of host finding (Hare et al. 1997). There is growing evidence that efficacy can be further improved in some insect species, including parasitoids, through additional temporally spaced learning experiences (Tully et al. 1994, Meller and Davis 1996, Kim et al. 2007, Campbell and Borden 2009). Combining sensitization exposure to host plants and ovipositioning experience on target host species can improve retention of parasitoids in target areas and increase host specificity. Associations with positive and negative experiences can help parasitoids learn to distinguish between similar odor blends, such as plants with and without infestations by host insects (Vet et al. 1990, 1998; Weiss et al. 2004). Other predatory insect species can be reared and conditioned in a similar manner, including lady beetles Latreille (Coleoptera: Coccinellidae) and lacewings Linnaeus (Neuroptera: Chrysopidae) (Wisenden et al. 1997, Reddy et al. 2004). Olfactory cues from both prey insects and host plants can be used to sensitize, attract, and retain predatory insects.
Naturally occurring generalist parasitoids can be induced to host-switch to invasive pest insect species through by blanketing infected crops with semiochemicals from host species or herbivore-induced plant volatiles (Barratt and Johnstone 2001). These will initially act as attractants into crop areas, where parasitoids can become conditioned to these novel hosts. Differential learning can be used to induce host-switching from a benign natural host to a related pest species.
Challenges Associated With Insect Learning and Pest Management
Host Shifting
Larval feeding experience can induce host preference changes for oviposition sites in female moths and for potential mating sites in male moths (Moreau et al. 2008, Anderson et al. 2013). This experience can override innate host preference hierarchies or override innate responses to deterrent compounds associated with larval food sources (Akhtar and Isman 2003, Moreau et al. 2008). The effects of larval experience can alter both host-finding and host-acceptance behavior (Olsson et al. 2006, Stamps and Davis 2006). This can result in either increased host fidelity or, under variable environmental conditions, can allow for adaptive responses that promote host-shifting and could facilitate sympatric speciation (Beltman et al. 2004, Beltman and Haccou 2005, Snell-Rood and Papaj 2009).
Chemical-Induced Interference
Studies of chemical-induced interference of insect learning and memory have primarily focused on experiments with beneficial hymenopteran insects, including honeybees (A. mellifera) and bumblebees (Bombus spp.). These studies have demonstrated that sublethal exposure to a variety of chemicals and pesticides can induce persistent disruptions in learning and memory (Aliouane et al. 2009, Frost et al. 2013, Siviter et al. 2018). Similar patterns of reduced learning and memory in honeybees can be attributed to parasitic infection and environmental conditions, including temperature extremes and changes in light exposure (Fitz-Earle and Sakaguchi 1986, Frost et al. 2011, Gegear et al. 2006, Kralj et al. 2007, Charbonneau et al. 2016). Pest management strategies must, therefore, take into consideration potential negative consequences of pesticides, physical barriers, and other treatment options on pollinators and other beneficial insects. Strategies should be examined on a case-by-case basis to ensure benefits in pest management efficacy outweigh potential risks.
Consequences of Agricultural Practices and Pest Management Strategies
Olfactory cues emanating from large-scale monoculture agricultural crops would rapidly become ubiquitous in the local environment, leading to sensitization for host odors and creating an ideal setting for learned acceptance by polyphagous insects (Smith and McSorley 2000, Potter and Held 2002, Teasdale et al. 2004, Marković 2013). Homogeneous forests due to replanting by commercial forestry industry pose a similar risk for learning by potential forest pest species (Jactel et al. 2005).
Because IPM efforts are not 100% effective, questions remain about the extent to which insects that are not killed can learn to avoid traps and lure odors that are used in monitoring or mass-trapping (Ebeling and Reierson 1969, Kain et al. 2013, Menda et al. 2013). Further study could help identify means to pest management practices to compensate, including alternating between two or more different lures. Insect learning of avoidance cues may also be closely tied to growing pesticide resistance. Survivors of pesticide exposure could learn to use olfactory, visual, or spatial cues associated with the experience to subsequently avoid or limit future exposure (Ebeling and Reierson 1969, Chareonviriyaphap et al. 1997, Rose et al. 2005, Cordeiro et al. 2013). Additionally, as insects become more resistant to a pesticide, they may also become habituated, reducing its efficacy as a deterrent. This may be particularly true in circumstances where pesticide applications were suboptimal due to dosage errors, missed treatments, or adverse weather conditions, and where insects were exposed to a negative but not lethal experience.
Insect Life History Effects
The efficacy of using insect learning in pest management strategies can differ among species with different life histories. Lifespan, feeding lifestyle (generalist vs specialist), and reproductive lifestyle are factors to be considered. Insect learning may be less useful in pest management strategies where insects involved are short-lived, multivoltine species. However, if long-lived species are involved as either pest or predator, learning may be much more relevant to pest management efforts. Subtle differences in reproductive lifestyles of parasitoid insects can have considerable differences in the types of cues learned. Parasitoids that arrest development in host species often use sensory cues associated with host species at earlier life stages to identify future host sites (Wäschke et al. 2013). In contrast, parasitoids that oviposit in hosts which will continue to develop and grow may also use cues associated with healthy host-food sources for identifying prospective hosts (Wäschke et al. 2013).
Receptivity to sensory cues and susceptibility to learning change over the life history of an insect (Browne and Withers 2002). Age, physiological condition, and environmental conditions can all influence reliance on learning and memory. Insects that have restricted access to food, mates, or suitable oviposition sites are likely to have reduced thresholds for or reduced reliance on learned chemical cues (Browne and Withers 2002). Desperation can supersede a learned hierarchy of preferences (Davis 2007, 2008). These insects may be more willing to risk imperfect matches to remembered stimuli and try novel hosts. Seasonal or circadian differences in host availability or environmental conditions can influence insect physiology and efficacy of learning (Decker et al. 2007, Garren et al. 2013). Learning is most productive when host availability and habitat conditions are stable during the lifetime of the insect but variable among generations (Segura et al. 2007, Smith and Raine 2014).
Limitations of Parasitoids and Predatory Insects as Control Agents
Parasitoids and predatory insects are growing in popularity as chemical-free alternative pest control measures (Tauber et al. 2000, Cônsoli and Grenier 2009, Parra 2009). In many cases, generalist parasitoids or predators are reared on alternate hosts or artificial media before being mass released. Efficacy can be improved by sensitizing newly emerging insects to their intended target hosts or to host-associated odors (Vet and Van Opzeeland 1984, Vet et al. 1995). Additionally, newly emerging insects can also be cross-conditioned to host-plant odors to encourage foraging within a crop area (Coaker and Cheah 1993, Storeck et al. 2000).
A critical first step is to identify candidate parasitoids or predatory insects (Mills 2005). Then suitable rearing conditions must be determined so that insects develop normally but are not conditioned to the wrong stimuli (Bigler 1989, Roitberg et al. 2001). A suitable sensory cue must then be applied at the appropriate point in the insect’s life history (e.g., at emergence or during a first oviposition experience) to ensure a persistent learned association. Assuming sufficient numbers of insects are available, timing for release is critical (Yang et al. 2006, Ovruski and Schliserman 2012, Pfab et al. 2018). If release occurs too early, no target hosts will be available, and parasitoids will disperse, or subsequent learning experiences will supersede the unreliable memory. If release occurs too late, the pest species will become too firmly established for parasitoids to effectively mitigate.
Host-insect and crop-plant semiochemicals can mediate host recognition in parasitoid wasps and improve host specificity, but effective semiochemical blends will differ depending upon parasitoids, host insects, and crops (Hare et al. 1993). Identifying and synthesizing effective semiochemical blends in sufficient quantities for pest management can be challenging (Hare et al. 1997). Costs associated with rearing, conditioning, and releasing parasitoids for pest control can also be a limiting factor for widespread use of this pest management strategy (Stevens et al. 2000).
Unintended Conditioning
The environment in which parasitoids and predatory insects are mass released or encouraged through attractant semiochemicals can become sources of information about risk for the pest insects upon which they prey (Wisenden et al. 1997). Bumblebees (Bombus spp.), cockroaches (Blattodea), and flies (Diptera) can learn to identify cues that signal predators, suggesting that risk aversion learning is common among many insect species. (McCall and Eaton 2001, Durisko and Dukas 2013, Dawson and Chittka 2014). In the absence of sufficient prey species, mass-released parasitoids may learn new olfactory associations that can override initial conditioning (Lewis et al. 1990, Grasman et al. 2001).
Future Directions for Pest Management
Direct Use of Insect Learning
Insect learning is already in use in many mass-release programs involving parasitoids. Improvements in efficacy, specificity, and memory duration could be achieved by introducing temporally spaced reinforcement conditioning experiences or supplementing host-odor associations with host-plant odors, particularly herbivory-induced plant odors (Steidle 1998, Ueno and Ueno 2005, Kim et al. 2007, Frost et al. 2012, Liu and Sakuma 2013, Giunti et al. 2015). Augmenting conditioning experiences with ferulic acid eicosyl ester, a component compound in the medicinal plant Rhodiola rosea Linnaeus (Saxifragales: Crassulaceae) used to improve human neurological function, could enhance memory function in diverse species (Michels et al. 2018). Conditioning parasitoids to multiple stimuli, including olfactory or visual cues associated with target hosts and plant crops, can have a synergetic effect on host specificity and encourage site fidelity within the crop area, reducing loss of parasitoids to dispersal (Lewis and Martin 1990, Gandolfi et al. 2003b, Giunti et al. 2015).
Endemic parasitoids and predatory insects could be reared and conditioned to prey on invasive species as they are introduced. In many cases, biological control efforts to eradicate invasive pest species have involved the use of introduced parasitoids or predators that might themselves become an invasive pest or prey on endemic benign species. Using conditioned endemic predators to control invasive pest could pose less risk to the existing ecosystem.
Indirect Use of Insect Learning
We can use our knowledge of how insects learn to develop more effective planting and pesticide use strategies and integrate the use of chemical repellents. Because repeated positive experiences can reinforce a conditioned response but experiencing a different or negative response following the same stimuli can reduce the reliability of information, crop rotation programs, and intercropping could disrupt associative learning of potential host plants (Papaj and Prokopy 1989, Meller and Davis 1996). Periodic changes in pesticide use could interrupt development of habituation or aversive learning by pest insects.
Studies on the effects of sublethal doses of chemical pesticides, primarily on honeybees, demonstrate long-term chemical-induced interference with insect learning (Aliouane et al. 2009, Frost et al. 2013, Samuelson et al. 2016, Klein et al. 2017, Tison et al. 2017, Siviter et al. 2018). Application of chemical pesticides during nonflowering periods (to reduce risk to pollinating insects) could result in similar learning and memory deficiencies in pest insects, preventing them from learning cues associated with suitability of novel crops as a potential host. Careful study is required to ensure that any potential benefits in limiting pest insect learning are not outweighed by risks to beneficial insects.
Use of sterile males is a useful strategy to mediate a variety of insects; however, results can vary among species or populations and are limited among insect species that mate with multiple partners (Fitz-Earle and Sakaguchi 1986). Many parasites and bacterial or viral pathogens are known to affect changes in host behavior, survival, resistance, and fecundity (Hurst and Jiggins 2000, Goodacre and Martin 2012). Bacteria could be identified or modified to restrict mushroom body development in host insects and thereby also limit potential for learning. Using sterile males as an initial bacterial vector, transmission could continue during mating, maternal transfer, or through contact with infected eggs, larvae, or adults (Ebbert 1995). As infection spreads through a target insect population, the ability of that insect species to learn and remember cues associated with food sources could be limited.
Beneficial Insects
Increased understanding of the impacts of pesticides and pest management methods on health and cognitive ability of pollinators and other beneficial insects can inform the development of targeted pest management strategies. Neonicotinoid and pyrethroid pesticides reduce cognitive function, learning, and memory in A. mellifera honeybees (Aliouane et al. 2009, Frost et al. 2013, Samuelson et al. 2016, Klein et al. 2017, Tison et al. 2017, Siviter et al. 2018). Timing of pesticide applications, choice of pesticides for use during each application, and potential alternatives to pesticides should be considered.
Honeybees are susceptible to a wide variety of parasites, including Varroa destructor Anderson and Trueman (Parasitiformes: Varroidae) mites. These mites parasitize primarily pupal stages of developing honeybees. Bees that experienced parasitism during pupal development become habituated to odor stimuli more quickly, become sensitized to new odors more slowly, and are less responsive to non-associative learning experiences (Kralj et al. 2007).
Biocontrol of Weed Plants
Classical biological control of invasive undesirable plant species (weeds) uses the intentional release of introduced phytophagous insect species as a biological control measure (Harris 1988). Prior to release, local regulators usually require that these insect species are tested for host acceptance and survival on a variety of commonly occurring native and crop plants; however, these represent only a small proportion of commonly occurring plant species in the release areas. Introduced insect species have been successful in reducing approximately one-third of the intended host weed-plant species; however, many introduced species also feed on native or crop plants (Harris 1988, Denslow and Johnson 2006). Dispersal hundreds or thousands of kilometers outside the intended control region or learning to use alternative nontarget host species are additional concerns (Pemberton 2000, Pratt and Center 2012, Suckling and Sforza 2014). We can reduce this risk by using associative conditioning with native phytophagous insect species. A large proportion of invasive plant species are suitable alternate hosts to native insect species (Bezemer et al. 2014). Inducing conditioned changes in host preference to favor feeding on invasive plant species could result in a host-shift that may be as effective as using introduced insect species, but with less risk of adding additional environmental stress to native plant species.
Conclusions
A first step in developing a long-term insect pest management strategy should be to acknowledge that insects can learn and will adapt. Integrated pest management strategies must evolve to remain effective. Insects can be both pest and benefactor in agricultural and forestry applications, disease vector control, and potentially in urban pest management. Learning by parasitoids and predatory insects can be mediated to help manage outbreaks of pest insects. Similarly, learning by phytophagous insects could be used to help control invasive plant species.
Current knowledge has given us the means to make significant gains in some areas of pest management, most notably using sensitization and associative conditioning of parasitoids for mass release against phytophagous pest insects. There is still much more that a knowledge of insect learning could contribute to biological control of pest insects (in agriculture, forestry, and urban settings), pest plants, and disease vectors.
Understanding mechanisms by which pest insects learn can inform the application of integrated pest management programs. It can give insight into means of modifying the conditions that allow insects to become pests. Growth in understanding of learning by beneficials, including predatory insects and pollinators, can help to offset detrimental effects of pesticides and toxic chemicals. Continued study of learning by parasitoids and predatory insects can lead to advances in pest management strategies that are less environmentally harmful. Understanding life history, habitat variability, and propensity to learn of insects of interest as pests or beneficials can help to identify potential areas of susceptibility and improve overall efficacy of pest management efforts. Continued growth in our knowledge of insect learning will contribute to our efforts to follow the basic tenant of integrated pest management, to ‘know your insect’. Learning is a journey, not a destination; even in insects.
Acknowledgments
We gratefully acknowledge the financial support of Atlantic Canada Opportunities Agency Atlantic Innovation Fund (197853), Canada Foundation for Innovation (22087), and Natural Sciences and Engineering Research Council of Canada (356109-2008 RGPIN and PGSD2-475743-2015).
References Cited
- Akhtar Y., and Isman M. B.. . 2003. Larval exposure to oviposition deterrents alters subsequent oviposition behavior in generalist, Trichoplusia ni and specialist, Plutella xylostella moths. J. Chem. Ecol. 29: 1853–1870. [DOI] [PubMed] [Google Scholar]
- Aliouane Y., El Hassani A. K., Gary V., Armengaud C., Lambin M., and Gauthier M.. 2009. Subchronic exposure of honeybees to sublethal doses of pesticides: effects on behavior. Environ. Toxicol. Chem. 28: 113–122. [DOI] [PubMed] [Google Scholar]
- van Alpern J. J. M., and Vet L. E. M.. . 1986. An evolutionary approach to host finding and selection, pp. 23–61. InWaage J. K., Greathead D. J. (eds.), Insect Parasitoids. Academic Press, London. [Google Scholar]
- Anderson P., Sadek M. M., Larsson M., Hansson B. S., and Thöming G.. . 2013. Larval host plant experience modulates both mate finding and oviposition choice in a moth. Anim. Behav. 85: 1169–1175. [Google Scholar]
- Armstrong J. D., de Belle J. S., Wang Z., and Kaiser K.. 1998. Metamorphosis of the mushroom bodies; large-scale rearrangements of the neural substrates for associative learning and memory in Drosophila. Learn. Mem. 5: 102–114. [PMC free article] [PubMed] [Google Scholar]
- Aso Y., Hattori D., Yu Y., Johnston R. M., Iyer N. A., Ngo T.-T., Dionne H., Abbott L., Axel R., Tanimoto H., and Rubin G. M.. . 2014. The neuronal architecture of the mushroom body provides a logic for associative learning. eLife. 3: e04577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avargues-Weber A., and Giurfa M.. . 2013. Conceptual learning by miniature brains. Proc Royal Soc. B. Biol. Sci. 280: 20131907–20131907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt B. I. P., and Johnstone P. D.. . 2001. Factors affecting parasitism by Microctonus aethiopoides (Hymenoptera: Braconidae) and parasitoid development in natural and novel host species. Bull. Entomol. Res. 91: 245–253. [DOI] [PubMed] [Google Scholar]
- Barron A. B. 2001. The life and death of Hopkins’ host-selection principle. J. Insect Behav. 14: 725–737. [Google Scholar]
- Barron A. B., and Corbet S. A.. . 1999. Preimaginal conditioning in Drosophila revisited. Anim. Behav. 58: 621–628. [DOI] [PubMed] [Google Scholar]
- Barron A. B., and Corbet S. A.. . 2000. Behavioural induction in Drosophila: timing and specificity. Entomol. Exp. Appl. 94: 159–171. [Google Scholar]
- Barron A. B., Hebets E. A., Cleland T. A., Fitzpatrick C. L., Hauber M. E., and Stevens J. R.. 2015. Embracing multiple definitions of learning. Trends Neurosci. 38: 405–407. [DOI] [PubMed] [Google Scholar]
- Bass C., Denholm I., Williamson M. S., and Nauen R.. 2015. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 121: 78–87. [DOI] [PubMed] [Google Scholar]
- de Belle J. S., and Heisenberg M.. . 1994. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 263: 692–695. [DOI] [PubMed] [Google Scholar]
- Beltman J. B., and Haccou P.. . 2005. Speciation through the learning of habitat features. Theor. Popul. Biol. 67: 189–202. [DOI] [PubMed] [Google Scholar]
- Beltman J. B., Haccou P., and ten Cate C.. 2004. Learning and colonization of new niches: a first step toward speciation. Evolution. 58: 35–46. [DOI] [PubMed] [Google Scholar]
- Benelli G., and Canale A.. . 2012. Learning of visual cues in the fruit fly parasitoid Psyttalia concolor (Szépligeti) (Hymenoptera: Braconidae). BioControl. 57: 767–777. [DOI] [PubMed] [Google Scholar]
- Bernays E. A. 1993. Aversion learning and feeding, pp. 1–17. InPapaj D. R. and Lewis A. C. (eds.), Insect learning. Springer US, Boston, MA. [Google Scholar]
- Bezemer T. M., Harvey J. A., and Cronin J. T.. 2014. Response of native insect communities to invasive plants. Annu. Rev. Entomol. 59: 119–141. [DOI] [PubMed] [Google Scholar]
- Bigler F. 1989. Quality assessment and control in entomophagous insects used for biological control. J. Appl. Entomol. 108: 390–400. [Google Scholar]
- Bjorksten T. A., and Hoffmann A. A.. . 1995. Effects of pre-adult and adult experience on host acceptance in choice and non-choice tests in two strains of Trichogramma. Entomol. Exp. Appl. 76: 49–58. [Google Scholar]
- Bjorksten T. A., and Hoffmann A. A.. . 1998. Persistence of experience effects in the parasitoid Trichogramma nr. brassicae. Ecol. Entomol. 23: 110–117. [Google Scholar]
- Blackiston D. J., Silva Casey E., and Weiss M. R.. . 2008. Retention of memory through metamorphosis: can a moth remember what it learned as a caterpillar? PLoS One 3: e1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonduriansky R., and Day T.. . 2009. Nongenetic inheritance and its evolutionary implications. Annu. Rev. Ecol. Evol. Syst. 40: 103–125. [Google Scholar]
- Brodie E. D. Jr, and Formanowicz D. R. Jr. 1981. Larvae of the predaceous diving beetle Dytiscus verticalis acquire an avoidance response to skin secretions of the newt Notophthalmus viridescens. Herpetologica. 37: 172–176. [Google Scholar]
- Brown C., and Laland K. N.. . 2003. Social learning in fishes: a review. Fish and Fisheries. 4: 280–288. [Google Scholar]
- Browne L. B., and Withers T. M.. . 2002. Time-dependent changes in the host-acceptance threshold of insects: implications for host specificity testing of candidate biological control agents. Biocontrol Sci. and Technol. 12: 677–693. [Google Scholar]
- Buatois A., Pichot C., Schultheiss P., Sandoz J.-C., Lazzari C. R., Chittka L., Avarguès-Weber A., and Giurfa M.. . 2017. Associative visual learning by tethered bees in a controlled visual environment. Sci. Reports. 7: 12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehlmann C., Hansson B. S., and Knaden M.. 2012. Desert ants learn vibration and magnetic landmarks. PLoS One 7: e33117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busto G. U., Cervantes-Sandoval I., and Davis R. L.. . 2010. Olfactory learning in Drosophila. Physiology. 25: 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S. A., and Borden J. H.. . 2009. Additive and synergistic integration of multimodal cues of both hosts and non-hosts during host selection by woodboring insects. Oikos. 118: 553–563. [Google Scholar]
- Capaldi E. A., Robinson G. E., and Fahrbach S. E.. . 1999. Neuroethology of spatial learning: the birds and the bees. Annu. Rev. Psychol. 50: 651–682. [DOI] [PubMed] [Google Scholar]
- Capaldi E. A., Smith A. D., Osborne J. L., Fahrbach S. E., Farris S. M., Reynolds D. R., Edwards A. S., Martin A., Robinson G. E., Poppy G. M., . et al. 2000. Ontogeny of orientation flight in the honeybee revealed by harmonic radar. Nature. 403: 537–540. [DOI] [PubMed] [Google Scholar]
- Carcaud J., Roussel E., Giurfa M., and Sandoz J. C.. 2009. Odour aversion after olfactory conditioning of the sting extension reflex in honeybees. J. Exp. Biol. 212: 620–626. [DOI] [PubMed] [Google Scholar]
- Carson R. 1962. Silent spring. Houghton Mifflin, Boston, MA. [Google Scholar]
- Caubet Y., and Jaisson P.. . 1991. A post-eclosion early learning involved in host recognition by Dinarmus basalis Rondani (Hymenoptera: Pteromalidae). Anim. Behav. 42: 977–980. [Google Scholar]
- Chareonviriyaphap T., Roberts D. R., Andre R. G., Harlan H. J., Manguin S., and Bangs M. J.. . 1997. Pesticide avoidance behavior in Anopheles albimanus, a malaria vector in the Americas. J. Am. Mosq. Control Assoc. 13: 171–183. [PubMed] [Google Scholar]
- Charbonneau L. R., Hillier N. K., Rogers R. E. L., Williams G. R., and Shutler D.. . 2016. Effects of Nosema apis, N. ceranae, and coinfections on honey bee (Apis mellifera) learning and memory. Sci. Reports. 6: 22626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittka L., and Leadbeater E.. . 2005. Social learning: public information in insects. Curr. Biol. 15: R869–R871. [DOI] [PubMed] [Google Scholar]
- Christiansen I. C., and Schausberger P.. . 2017. Interference in early dual-task learning by predatory mites. Anim. Behav. 133: 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coaker T. H., and Cheah C. A.. . 1993. Conditioning as a factor in parasitoid host plant preference. Biocontrol Sci. Technol. 3: 277–283. [Google Scholar]
- Collett T. S. 2008. Insect behaviour: learning for the future. Curr. Biol. 18: R131–R134. [DOI] [PubMed] [Google Scholar]
- Coolen I., Dangles O., and Casas J.. 2005. Social learning in noncolonial insects? Curr. Biol. 15: 1931–1935. [DOI] [PubMed] [Google Scholar]
- Corbet S. A. 1985. Insect chemosensory responses: a chemical legacy hypothesis. Ecol. Entomol. 10: 143–153. [Google Scholar]
- Cônsoli F. L., and Grenier S.. . 2009. In vitro rearing of egg parasitoids, pp. 293–313. InCônsoli F. L., Parra J. R. P., Zucchi R. A. (eds.), Egg parasitoids in agroecosystems with emphasis on trichogramma, progress in biological control. Springer Netherlands, Dordrecht. [Google Scholar]
- Cordeiro E. M. G., de Moura I. L. T., Fadini M. A. M., and Guedes R. N. C.. . 2013. Beyond selectivity: are behavioral avoidance and hormesis likely causes of pyrethroid-induced outbreaks of the southern red mite Oligonychus ilicis? Chemosphere. 93: 1111–1116. [DOI] [PubMed] [Google Scholar]
- Craighead F. C. 1921. Hopkins host-selection principle as related to certain cerambycid beetles. J. Agric. Res. 22: 189–220. [Google Scholar]
- Craighead F. C. 1923. Popular and practical entomology: the host selection principle as advanced by Walsh. Can. Entomol. 55: 76–79. [Google Scholar]
- Cunningham J. P., Jallow M. F. A., Wright D. J., and Zalucki M. P.. 1998. Learning in host selection in Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). Anim. Behav. 55: 227–234. [DOI] [PubMed] [Google Scholar]
- Cunningham J. P., Zalucki M. P., and West S. A.. . 1999. Learning in Helicoverpa armigera (Lepidoptera: Noctuidae): a new look at the behaviour and control of a polyphagous pest. Bull. Entomol. Res. 89: 201–207. [Google Scholar]
- Cunningham J. P., West S. A., and Zalucki M. P.. 2001. Host selection in phytophagous insects: a new explanation for learning in adults. Oikos. 95: 537–543. [Google Scholar]
- Dawson E. H., and Chittka L.. . 2014. Bumblebees (Bombus terrestris) use social information as an indicator of safety in dangerous environments. Proc Royal Soc. B.: Biol. Sci. 281: 20133174–20133174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly K. C., Christensen T. A., Lei H., Smith B. H., and Hildebrand J. G.. 2004. Learning modulates the ensemble representations for odors in primary olfactory networks. Proc. Natl. Acad. Sci. U. S. A. 101: 10476–10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. M. 2007. Preference or desperation? Distinguishing between the natal habitat’s effects on habitat choice. Anim. Behav. 74: 111–119. [Google Scholar]
- Davis J. M. 2008. Patterns of variation in the influence of natal experience on habitat choice. Q. Rev. Biol. 83: 363–380. [DOI] [PubMed] [Google Scholar]
- Decker S., McConnaughey S., and Page T. L.. 2007. Circadian regulation of insect olfactory learning. Proc. Natl. Acad. Sci. U. S. A. 104: 15905–15910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denslow J. S., and Johnson M. T.. . 2006. Biological control of tropical weeds: research opportunities in plant-herbivore interactions. Biotropica. 38: 139–142. [Google Scholar]
- Desurmont G. A., Guiguet A., and Turlings T. C. J.. . 2018. Invasive insect herbivores as disrupters of chemically-mediated tritrophic interactions: effects of herbivore density and parasitoid learning. Biol. Invasions. 20: 195–206. [Google Scholar]
- Devaud J. M., Acebes A., and Ferrús A.. 2001. Odor exposure causes central adaptation and morphological changes in selected olfactory glomeruli in Drosophila. J. Neurosci. 21: 6274–6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez L., Deneubourg J.-L., Hoebeke L., and Detrain C.. . 2011. Orientation in corpse-carrying ants: memory or chemical cues? Anim. Behav. 81: 1171–1176. [Google Scholar]
- Drost Y. C., Lewis W. J., Zanen P. O., and Keller M. A.. . 1986. Beneficial arthropod behavior mediated by airborne semiochemicals. J. Chem. Ecol. 12: 1247–1262. [DOI] [PubMed] [Google Scholar]
- Du Y., Poppy G. M., Powell W., and Wadhams L. J.. . 1997. Chemically mediated associative learning in the host foraging behavior of the aphid parasitoid Aphidius ervi (Hymenoptera: Braconidae). J. Insect Behav. 10: 509–522. [Google Scholar]
- Dukas R. 2008. Evolutionary biology of insect learning. Annu. Rev. Entomol. 53: 145–160. [DOI] [PubMed] [Google Scholar]
- Dunlap A. S., Nielsen M. E., Dornhaus A., and Papaj D. R.. 2016. Foraging bumble bees weigh the reliability of personal and social information. Curr. Biol. 26: 1195–1199. [DOI] [PubMed] [Google Scholar]
- Durisko Z., and Dukas R.. . 2013. Attraction to and learning from social cues in fruitfly larvae. Proc Royal Soc. B. Biol. Sci. 280: 20131398–20131398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer A. G., Rosa M. G., and Reser D. H.. 2008. Honeybees can recognise images of complex natural scenes for use as potential landmarks. J. Exp. Biol. 211: 1180–1186. [DOI] [PubMed] [Google Scholar]
- Ebbert M. A. 1995. Variable effects of crowding on Drosophila hosts of male-lethal and non-male-lethal spiroplasmas in laboratory populations. Heredity. 74: 227. [DOI] [PubMed] [Google Scholar]
- Ebeling W., and Reierson D. A.. . 1969. The cockroach learns to avoid insecticides. Calif Agr. 23: 12–15. [Google Scholar]
- Ebeling W., Wagner R. E., and Reierson D. A.. 1966. Influence of repellency on the efficacy of blatticides. I. Learned modification of behavior of the German cockroach. J. Econ. Entomol. 59: 1374–1388. [DOI] [PubMed] [Google Scholar]
- El Jundi B., Foster J. J., Khaldy L., Byrne M. J., Dacke M., and Baird E.. 2016. A snapshot-based mechanism for celestial orientation. Curr. Biol. 26: 1456–1462. [DOI] [PubMed] [Google Scholar]
- Enjin A., Zaharieva E. E., Frank D. D., Mansourian S., Suh G. S., Gallio M., and Stensmyr M. C.. 2016. Humidity sensing in Drosophila. Curr. Biol. 26: 1352–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst U. R., Van Hiel M. B., Depuydt G., Boerjan B., De Loof A., and Schoofs L.. . 2015. Epigenetics and locust life phase transitions. J. Exp. Biol. 218: 88–99. [DOI] [PubMed] [Google Scholar]
- Faber T., Joerges J., and Menzel R.. 1999. Associative learning modifies neural representations of odors in the insect brain. Nat. Neurosci. 2: 74–78. [DOI] [PubMed] [Google Scholar]
- Farina W. M., Gruter C., and Diaz P. C.. . 2005. Social learning of floral odours inside the honeybee hive. Proc Royal Soc. B. Biol. Sci. 272: 1923–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqui T., Robinson K., Vaessin H., and Smith B. H.. 2003. Modulation of early olfactory processing by an octopaminergic reinforcement pathway in the honeybee. J. Neurosci. 23: 5370–5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris S. M., Robinson G. E., and Fahrbach S. E.. 2001. Experience- and age-related outgrowth of intrinsic neurons in the mushroom bodies of the adult worker honeybee. J. Neurosci. 21: 6395–6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala A. 2007. Olfaction and olfactory learning in Drosophila: recent progress. Curr. Opin. Neurobiol. 17: 720–726. [DOI] [PubMed] [Google Scholar]
- Finch S., and Collier R. H.. . 2000. Host-plant selection by insects - a theory based on “appropriate/inappropriate landings” by pest insects of cruciferous plants. Entomol. Exp. Appl. 96: 91–102. [Google Scholar]
- Fitz-Earle M., and Sakaguchi B.. . 1986. Sex ratio distortion in populations and its possible role in insect suppression: experimental studies with strains of Drosophila melanogaster carrying cytoplasmically-inherited male-killing spiroplasmas. Japanese J. Genetics. 61: 447–460. [Google Scholar]
- Franks N. R., and Richardson T.. . 2006. Teaching in tandem-running ants. Nature. 439: 153. [DOI] [PubMed] [Google Scholar]
- Frost E. H., Shutler D., and Hillier N. K.. 2011. Effects of cold immobilization and recovery period on honeybee learning, memory, and responsiveness to sucrose. J. Insect Physiol. 57: 1385–1390. [DOI] [PubMed] [Google Scholar]
- Frost E. H., Shutler D., and Hillier N. K.. 2012. The proboscis extension reflex to evaluate learning and memory in honeybees (Apis mellifera): some caveats. Naturwissenschaften. 99: 677–686. [DOI] [PubMed] [Google Scholar]
- Frost E. H., Shutler D., and Hillier N. K.. . 2013. Effects of fluvalinate on honey bee learning, memory, responsiveness to sucrose, and survival. J. Exp. Biol. 216: 2931–2938. [DOI] [PubMed] [Google Scholar]
- Galef B. G., Jr 1995. Why behaviour patterns that animals learn socially are locally adaptive. Anim. Behav. 49: 1325–1334. [Google Scholar]
- Galizia C. G., and Sachse S.. . 2010. Odor coding in insects, pp. 35–70. InMenini A., (ed.), The neurobiology of olfaction. CRC Press, New York, NY. [PubMed] [Google Scholar]
- Gandolfi M., Mattiacci L., and Dorn S.. . 2003a. Preimaginal learning determines adult response to chemical stimuli in a parasitic wasp. Proc Royal Soc. B. Biol. Sci. 270: 2623–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandolfi M., Mattiacci L., and Dorn S.. 2003b. Mechanisms of behavioral alterations of parasitoids reared in artificial systems. J. Chem. Ecol. 29: 1871–1887. [DOI] [PubMed] [Google Scholar]
- Garren M. V., Sexauer S. B., and Page T. L.. 2013. Effect of circadian phase on memory acquisition and recall: operant conditioning vs. classical conditioning. PLoS One 8: e58693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegear R. J., Otterstatter M. C., and Thomson J. D.. . 2006. Bumble-bee foragers infected by a gut parasite have an impaired ability to utilize floral information. Proc. Royal Soc. B Biol. Sci. 273: 1073–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber B., and Stocker R. F.. . 2007. The Drosophila larva as a model for studying chemosensation and chemosensory learning: a review. Chem. Senses 32: 65–89. [DOI] [PubMed] [Google Scholar]
- Griffin A. S. 2004. Social learning about predators: a review and prospectus. Anim Learn Behav. 32: 131–140. [DOI] [PubMed] [Google Scholar]
- Giunti G., Canale A., Messing R. H., Donati E., Stefanini C., Michaud J. P., and Benelli G.. . 2015. Parasitoid learning: current knowledge and implications for biological control. Biol. Control. 90: 208–219. [Google Scholar]
- Giurfa M. 2012. Social learning in insects: a higher-order capacity? Frontiers Behav. Neurosci. 6: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodacre S. L., and Martin O. Y.. . 2012. Modification of insect and arachnid behaviours by vertically transmitted endosymbionts: infections as drivers of behavioural change and evolutionary novelty. Insects. 3: 246–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasman J., van Herwaarden O. A., Hemerik L., and van Lenteren J. C.. 2001. A two-component model of host-parasitoid interactions: determination of the size of inundative releases of parasitoids in biological pest control. Math. Biosci. 169: 207–216. [DOI] [PubMed] [Google Scholar]
- Gross H. R., Lewis W. J., Jones R. L., and Nordlund D. A.. . 1975. Kairomones and their use for management of entomophagous insects: III. Stimulation of Trichogramma achaeae, T. pretiosum, and Microplitis croceipes with host-seeking stimuli at time of release to improve their efficiency. J. Chem. Ecol. 1: 431–438. [Google Scholar]
- Grossmann K. E. 1973. Continuous, fixed-ratio, and fixed-interval reinforcement in honey bees. J. Exp. Anal. Behav. 20: 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer M., and Menzel R.. . 1998. Multiple sites of associative odor learning as revealed by local brain microinjections of octopamine in honeybees. Learn. Mem. 5: 146–156. [PMC free article] [PubMed] [Google Scholar]
- Hare J. D., Millar J. G., and Luck R. F.. . 1993. A caffeic acid ester mediates host recognition by a parasitic wasp. Naturwissenschaften. 80: 92–94. [Google Scholar]
- Hare J. D., Morgan D. J. W., and Nguyun T.. . 1997. Increased parasitization of California red scale in the field after exposing its parasitoid, Aphytis melinus, to a synthetic kairomone. Entomol. Exp. Appl. 82: 73–81. [Google Scholar]
- Harris P. 1988. Environmental impact of weed-control insects. Biosci. 38: 542–548. [Google Scholar]
- Heinrich B. 1979. Foraging strategies of caterpillars. Oecologia. 42: 325–337. [DOI] [PubMed] [Google Scholar]
- Hendel T., Michels B., Neuser K., Schipanski A., Kaun K., Sokolowski M. B., Marohn F., Michel R., Heisenberg M., and Gerber B.. . 2005. The carrot, not the stick: appetitive rather than aversive gustatory stimuli support associative olfactory learning in individually assayed Drosophila larvae. J. Compar. Physiol. A. 191: 265–279. [DOI] [PubMed] [Google Scholar]
- Hérard F., Keller M. A., Lewis W. J., and Tumlinson J. H.. . 1988. Beneficial arthropod behavior mediated by airborne semiochemicals. J. Chem. Ecol. 14: 1583–1596. [DOI] [PubMed] [Google Scholar]
- Hershberger W. A., and Smith M. P.. . 1967. Conditioning in Drosophila melanogaster. Anim. Behav. 15: 259–262. [DOI] [PubMed] [Google Scholar]
- Hopkins A. D. 1916. Economic investigations of the scolytid bark and timber beetles of North America. US Dept. Agric. Program of Work. 353: 353. [Google Scholar]
- Hopkins A. D. 1917. Contribution to discussion. J. Econ. Entomol. 10: 92–93. [Google Scholar]
- Hoppitt W., and Laland K. N.. . 2008. Chapter 3 Social processes influencing learning in animals: a review of the evidence. Adv. Study Behav. 38: 105–165. [Google Scholar]
- Horwith B. 1985. A role for intercropping in modern agriculture. BioScience. 35: 286–291. [Google Scholar]
- Hoskins W. M., Borden A. D., and Michelbacher A. E.. . 1939. Recommendations for a more discriminating use of insecticides, pp. 119–123. InProceedings of Sixth Pacific Science Congress 24 July-12 August 1939, University of California, Berkeley, Stanford University, and San Francisco. [Google Scholar]
- Hurst G., and Jiggins F. M.. . 2000. Male-killing bacteria in insects: mechanisms, incidence, and implications. Emerg. Infect. Dis. 6: 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- I’Anson Price R., Dulex N., Vial N., Vincent C., and Grüter C.. . 2019. Honeybees forage more successfully without the “dance language” in challenging environments. Sci. Adv. 5: eaat0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jactel H., Brockerhoff E., and Duelli P.. . 2005. A test of the biodiversity-stability theory: Meta-analysis of tree species diversity effects on insect pest infestations, and re-examination of responsible factors, pp. 235–262. InScherer-Lorenzen M., Körner C., and Schulze E.-D. (eds.), Forest diversity and function: temperate and boreal systems. Springer Berlin Heidelberg, Berlin, Heidelberg, Germany. [Google Scholar]
- Jaenike J., and Holt R. D.. . 1991. Genetic variation for habitat preference: evidence and explanations. Am. Nat. 137: S67–S90. [Google Scholar]
- Jones P. L., Ryan M. J., and Chittka L.. . 2015. The influence of past experience with flower reward quality on social learning in bumblebees. Anim. Behav. 101: 11–18. [Google Scholar]
- Jørgensen K., Stranden M., Sandoz J. C., Menzel R., and Mustaparta H.. 2007. Effects of two bitter substances on olfactory conditioning in the moth Heliothis virescens. J. Exp. Biol. 210: 2563–2573. [DOI] [PubMed] [Google Scholar]
- Kain P., Boyle S. M., Tharadra S. K., Guda T., Pham C., Dahanukar A., and Ray A.. 2013. Odour receptors and neurons for DEET and new insect repellents. Nature. 502: 507–512. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kaiser L., and De Jong R.. . 1993. Multi-odour memory influenced by learning order. Behav. Proc. 30: 175–183. [DOI] [PubMed] [Google Scholar]
- Kaiser L., Pham-Delegue M. H., and Masson C.. . 1989. Behavioural study of plasticity in host preferences of Trichogramma maidis (Hym.: Trichogrammatidae). Physiol. Entomol. 14: 53–60. [Google Scholar]
- Kawaguchi L. G., Ohashi K., and Toquenaga Y.. . 2006. Do bumble bees save time when choosing novel flowers by following conspecifics? Funct. Ecol. 20: 239–244. [Google Scholar]
- Keleman K., Vrontou E., Krüttner S., Yu J. Y., Kurtovic-Kozaric A., and Dickson B. J.. 2012. Dopamine neurons modulate pheromone responses in Drosophila courtship learning. Nature. 489: 145–149. [DOI] [PubMed] [Google Scholar]
- Kellert S. R. 1993. Values and perceptions of invertebrates. Conserv. Biol. 7: 845–855. [Google Scholar]
- Kells S. A., Lee C. Y., and Robinson W. H.. . 2005. Bait aversion by German cockroaches (Dictyoptera: Blattellidae): the influence and interference of nutrition, pp. 419–422. InProceedings of the Fifth International Conference on Urban Pests, 10-13 July 2005. International Conference on Urban Pests (ICUP), Singapore. [Google Scholar]
- Kijimoto T., and Moczek A. P.. . 2016. Hedgehog signaling enables nutrition-responsive inhibition of an alternative morph in a polyphenic beetle. Proc. Natl. Acad. Sci. U. S. A. 113: 5982–5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.-C., Lee H.-G., and Han K.-A.. . 2007. Classical reward conditioning in Drosophila melanogaster. Genes Brain Behav. 6: 201–207. [DOI] [PubMed] [Google Scholar]
- Kirchner W. H., Dreller C., and Towne W. F.. . 1991. Hearing in honeybees: operant conditioning and spontaneous reactions to airborne sound. J. Compar. Physiol. A. 168: 85–89. [Google Scholar]
- Klein S., Cabirol A., Devaud J.-M., Barron A. B., and Lihoreau M.. . 2017. Why bees are so vulnerable to environmental stressors. Trends Ecol. Evol. 32: 268–278. [DOI] [PubMed] [Google Scholar]
- Kleineidam C. J., Ruchty M., Casero-Montes Z. A., and Roces F.. 2007. Thermal radiation as a learned orientation cue in leaf-cutting ants (Atta vollenweideri). J. Insect Physiol. 53: 478–487. [DOI] [PubMed] [Google Scholar]
- Knörzer H., Graeff-Hönninger S., Guo B., Wang P., and Claupein W.. . 2009. The rediscovery of intercropping in China: a traditional cropping system for future Chinese agriculture – A review, pp. 13–44. InLichtfouse E. (ed.), Climate change, intercropping, pest control and beneficial microorganisms. Springer Netherlands, Dordrecht, Netherlands. [Google Scholar]
- Kogan M. 1998. Integrated pest management: historical perspectives and contemporary developments. Annu. Rev. Entomol. 43: 243–270. [DOI] [PubMed] [Google Scholar]
- Kralj J., Brockmann A., Fuchs S., and Tautz J.. . 2007. The parasitic mite Varroa destructor affects non-associative learning in honey bee foragers, Apis mellifera L. J. Compar. Physiol. A. 193: 363–370. [DOI] [PubMed] [Google Scholar]
- Larkin A., Karak S., Priya R., Das A., Ayyub C., Ito K., Rodrigues V., and Ramaswami M.. 2010. Central synaptic mechanisms underlie short-term olfactory habituation in Drosophila larvae. Learn. Mem. 17: 645–653. [DOI] [PubMed] [Google Scholar]
- Leadbeater E. A., and Chittka L.. . 2009. Social information use in foraging insects, pp. 135–146. InJarau S. and Hrncir M. (eds.), Food exploitation by social insects: ecological, behavioral, and theoretical approaches. CRC Press, Boca Raton, FL. [Google Scholar]
- Leadbeater E., and Chittka L.. . 2007. Social learning in insects–from miniature brains to consensus building. Curr. Biol. 17: R703–R713. [DOI] [PubMed] [Google Scholar]
- Leadbeater E., and Dawson E. H.. . 2017. A social insect perspective on the evolution of social learning mechanisms. Proc. Nat. Acad. Sci. 114: 7838–7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis W. J., Jones R. L., Nordlund D. A., and Gross H. R.. . 1975. Kairomones and their use for management of entomophagous insects: II. Mechanisms causing increase in rate of parasitization by Trichogramma spp. J. Chem. Ecol. 1: 349–360. [Google Scholar]
- Lewis W. J., and Martin W. R. Jr. 1990. Semiochemicals for use with parasitoids: status and future. J. Chem. Ecol. 16: 3067–3089. [DOI] [PubMed] [Google Scholar]
- Lewis W. J., Tumlinson J. H., and Krasnoff S.. . 1991. Chemically mediated associative learning: An important function in the foraging behavior of Microplitis croceipes (Cresson). J. Chem. Ecol. 17: 1309–1325. [DOI] [PubMed] [Google Scholar]
- Lewis W. J., Vet L. E. M., Tumlinson J. H., Van Lenteren J. C., and Papaj D. R.. . 1990. Variations in parasitoid foraging behavior: essential element of a sound biological control theory. Environ. Entomol. 19: 1183–1193. [Google Scholar]
- Lihoreau M., and Rivault C.. . 2011. Local enhancement promotes cockroach feeding aggregations. PLoS One 6: e22048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.-L., and Sakuma M.. . 2013. Olfactory conditioning with single chemicals in the German cockroach, Blattella germanica (Dictyoptera: Blattellidae). Appl. Entomol. and Zool. 48: 387–396. [Google Scholar]
- Loke W. H., and Ashley T. R.. . 1984. Potential uses of kairomones for behavioral manipulation of Cotesia marginiventris (Cresson). J. Chem. Ecol. 10: 1377–1384. [DOI] [PubMed] [Google Scholar]
- Marković D. 2013. Crop diversification affects biological pest control. Agro-knowledge J. 14: 449. [Google Scholar]
- Martin T., Kamal A., Gogo E., Saidi M., Delétré E., Bonafos R., Simon S., and Ngouajio M.. 2014. Repellent effect of alphacypermethrin-treated netting against Bemisia tabaci (Hemiptera: Aleyrodidae). J. Econ. Entomol. 107: 684–690. [DOI] [PubMed] [Google Scholar]
- McCall P. J., and Eaton G.. . 2001. Olfactory memory in the mosquito Culex quinquefasciatus. Med. Vet. Entomol. 15: 197–203. [DOI] [PubMed] [Google Scholar]
- McCall P. J., and Kelly D. W.. . 2002. Learning and memory in disease vectors. Trends Parasitol. 18: 429–433. [DOI] [PubMed] [Google Scholar]
- McCall P. J., Turlings T. C. J., Lewis W. J., and Tumlinson J. H.. . 1993. Role of plant volatiles in host location by the specialist parasitoid Microplitis croceipes Cresson (Braconidae: Hymenoptera). J. Insect Behav. 6: 625–639. [Google Scholar]
- Meller V. H., and Davis R. L.. . 1996. Biochemistry of insect learning: lessons from bees and flies. Insect Biochem. Mol. Biol. 26: 327–335. [DOI] [PubMed] [Google Scholar]
- Menda G., Uhr J. H., Wyttenbach R. A., Vermeylen F. M., Smith D. M., Harrington L. C., and Hoy R. R.. 2013. Associative learning in the dengue vector mosquito, Aedes aegypti: avoidance of a previously attractive odor or surface color that is paired with an aversive stimulus. J. Exp. Biol. 216: 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel R. 2012. The honeybee as a model for understanding the basis of cognition. Nat. Rev. Neurosci. 13: 758. [DOI] [PubMed] [Google Scholar]
- Menzel R., and Muller U.. . 1996. Learning and memory in honeybees: from behavior to neural substrates. Annu. Rev. Neurosci. 19: 379–404. [DOI] [PubMed] [Google Scholar]
- Michels B., Zwaka H., Bartels R., Lushchak O., Franke K., Endres T., Fendt M., Song I., Bakr M., Budragchaa T., . et al. 2018. Memory enhancement by ferulic acid ester across species. Sci. Adv. 4: eaat6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills N. 2005. Selecting effective parasitoids for biological control introductions: codling moth as a case study. Biol. Control. 34: 274–282. [Google Scholar]
- Molet M., Chittka L., and Raine N. E.. 2009. How floral odours are learned inside the bumblebee (Bombus terrestris) nest. Naturwissenschaften. 96: 213–219. [DOI] [PubMed] [Google Scholar]
- Moreau J., Rahme J., Benrey B., and Thiery D.. 2008. Larval host plant origin modifies the adult oviposition preference of the female European grapevine moth Lobesia botrana. Naturwissenschaften. 95: 317–324. [DOI] [PubMed] [Google Scholar]
- N’Guessan R., Corbel V., Akogbéto M., and Rowland M.. . 2007. Reduced efficacy of insecticide-treated nets and indoor residual spraying for malaria control in pyrethroid resistance area, Benin. Emerg. Infect. Dis. 13: 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano H., Hirano T., and Balaban E.. . 2000. Learning and memory. Proc. Nat. Acad. Sci. 97: 12403–12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson P.-O. C., Anderbrant O., and Löfstedt C.. . 2006. Experience influences oviposition behaviour in two pyralid moths, Ephestia cautella and Plodia interpunctella. Anim. Behav. 72: 545–551. [Google Scholar]
- Ovruski S. M., and Schliserman P.. . 2012. Biological control of tephritid fruit flies in Argentina: historical review, current status, and future trends for developing a parasitoid mass-release program. Insects. 3: 870–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra J. R. P. 2009. Mass rearing of egg parasitoids for biological control programs, pp. 267–292. InConsoli F. L., Parra J. R. P., and Zucchi R. A. (eds.), Egg parasitoids in agroecosystems with emphasis on trichogramma. Springer Netherlands, Dordrecht, Netherlands. [Google Scholar]
- Papaj D. R., and Prokopy R. J.. . 1989. Ecological and evolutionary aspects of learning in phytophagous insects. Ann. Rev. Entomol. 34: 315–350. [Google Scholar]
- Pemberton R. W. 2000. Predictable risk to native plants in weed biological control. Oecologia. 125: 489–494. [DOI] [PubMed] [Google Scholar]
- Pfab F., Stacconi M. V. R., Anfora G., Grassi A., Walton V., and Pugliese A.. . 2018. Optimized timing of parasitoid release: a mathematical model for biological control of Drosophila suzukii. Theor. Ecol. 11: 489–501. [Google Scholar]
- Pfennig D. W., Wund M. A., Snell-Rood E. C., Cruickshank T., Schlichting C. D., and Moczek A. P.. 2010. Phenotypic plasticity’s impacts on diversification and speciation. Trends Ecol. Evol. 25: 459–467. [DOI] [PubMed] [Google Scholar]
- Potter D. A., and Held D. W.. . 2002. Biology and management of the Japanese beetle. Annu. Rev. Entomol. 47: 175–205. [DOI] [PubMed] [Google Scholar]
- Pratt P. D., and Center T. D.. . 2012. Biocontrol without borders: the unintended spread of introduced weed biological control agents. BioControl. 57: 319–329. [Google Scholar]
- Rains G. C., Tomberlin J. K., and Kulasiri D.. 2008. Using insect sniffing devices for detection. Trends Biotechnol. 26: 288–294. [DOI] [PubMed] [Google Scholar]
- Rasekh A., Michaud J. P., Allahyari H., and Sabahi Q.. . 2010. The foraging behavior of Lysiphlebus fabarum (Marshall), a thelytokous parasitoid of the black bean aphid in Iran. J. Insect Behav. 23: 165–179. [Google Scholar]
- Reddy G. V. P., Tabone E., and Smith M. T.. . 2004. Mediation of host selection and oviposition behavior in the diamondback moth Plutella xylostella and its predator Chrysoperla carnea by chemical cues from cole crops. Biol. Control. 29: 270–277. [Google Scholar]
- Reisen W. K. 1975. Intraspecific competition in Anopheles stephensi Liston. Mosquito News. 35: 473–481. [Google Scholar]
- Rietdorf K., and Steidle J. L. M.. . 2002. Was Hopkins right? Influence of larval and early adult experience on the olfactory response in the granary weevil Sitophilus granarius (Coleoptera, Curculionidae). Physiol. Entomol. 27: 223–227. [Google Scholar]
- Roberts D. 2012. Responses of three species of mosquito larvae to the presence of predatory dragonfly and damselfly larvae. Entomol. Exp. Appl. 145: 23–29. [Google Scholar]
- Roitberg B. D., Boivin G., and Vet L. E. M.. . 2001. Fitness, parasitoids, and biological control: an opinion. The Canadian Entomologist. 133: 429–438. [Google Scholar]
- Rose D., Leather S. R., and Matthews G. A.. . 2005. Recognition and avoidance of insecticide-treated Scots Pine (Pinus sylvestris) by Hylobius abietis (Coleoptera: Curculionidae): implications for pest management strategies. Agric. For. Entomol. 7: 187–191. [Google Scholar]
- Sachse S., Rueckert E., Keller A., Okada R., Tanaka N. K., Ito K., and Vosshall L. B.. 2007. Activity-dependent plasticity in an olfactory circuit. Neuron. 56: 838–850. [DOI] [PubMed] [Google Scholar]
- Samuelson E. E. W., Chen-Wishart Z. P., Gill R. J., and Leadbeater E.. . 2016. Effect of acute pesticide exposure on bee spatial working memory using an analogue of the radial-arm maze. Sci. Rep. 6: 38957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarin S., and Dukas R.. . 2009. Social learning about egg-laying substrates in fruitflies. Proc Royal Soc. B. Biol. Sci. 276: 4323–4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saumweber T., Cano C., Klessen J., Eichler K., Fendt M., and Gerber B.. 2014. Immediate and punitive impact of mechanosensory disturbance on olfactory behaviour of larval Drosophila. Biol. Open. 3: 1005–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saverschek N., Herz H., Wagner M., and Roces F.. . 2010. Avoiding plants unsuitable for the symbiotic fungus: learning and long-term memory in leaf-cutting ants. Anim. Behav. 79: 689–698. [Google Scholar]
- Saverschek N., and Roces F.. . 2011. Foraging leafcutter ants: olfactory memory underlies delayed avoidance of plants unsuitable for the symbiotic fungus. Anim. Behav. 82: 453–458. [Google Scholar]
- Scherer S. 2003. Olfactory learning in individually assayed Drosophila larvae. Learn. Mem. 10: 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroll C., Riemensperger T., Bucher D., Ehmer J., Völler T., Erbguth K., Gerber B., Hendel T., Nagel G., Buchner E., et al. 2006. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr. Biol. 16: 1741–1747. [DOI] [PubMed] [Google Scholar]
- Schwaerzel M., Monastirioti M., Scholz H., Friggi-Grelin F., Birman S., and Heisenberg M.. 2003. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J. Neurosci. 23: 10495–10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura D. F., Viscarret M. M., Carabajal Paladino L. Z., Ovruski S. M., and Cladera J. L.. . 2007. Role of visual information and learning in habitat selection by a generalist parasitoid foraging for concealed hosts. Anim. Behav. 74: 131–142. [Google Scholar]
- Shanower T. G., Romeis J., and Minja E. M.. 1999. Insect pests of pigeonpea and their management. Annu. Rev. Entomol. 44: 77–96. [DOI] [PubMed] [Google Scholar]
- Shephard A. M., Aksenov V., and Rollo C. D.. . 2018. Conspecific mortality cues mediate associative learning in crickets, Acheta domesticus (Orthoptera: Gryllidae). J. Orthoptera Res. 27: 187–192. [Google Scholar]
- Siviter H., Koricheva J., Brown M. J. F., and Leadbeater E.. 2018. Quantifying the impact of pesticides on learning and memory in bees. J. Appl. Ecol. 55: 2812–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaa E. J., Wassenberg J., and Biesmeijer J. C.. . 2003. The use of field-based social information in eusocial foragers: local enhancement among nestmates and heterospecifics in stingless bees. Ecol. Entomol. 28: 369–379. [Google Scholar]
- Smith H. A., and McSorley R.. . 2000. Intercropping and pest management: a review of major concepts. Am. Entomol. 46: 154–161. [Google Scholar]
- Smith K. E., and Raine N. E.. . 2014. A comparison of visual and olfactory learning performance in the bumblebee Bombus terrestris. Behav. Ecol. Sociobiol. 68: 1549–1559. [Google Scholar]
- Smith R. F., and Allen W. W.. . 1954. Insect control and the balance of nature. Sci. Am. 190: 38–42. [Google Scholar]
- Smolla M., Alem S., Chittka L., and Shultz S.. . 2016. Copy when uncertain: bumblebees rely on social information when rewards are highly variable. Biol. Letters. 12: 20160188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell‐Rood E. C., and Papaj D. R.. . 2009. Patterns of phenotypic plasticity in common and rare environments: a study of host use and color learning in the cabbage white butterfly Pieris rapae. Am. Nat. 173: 615–631. [DOI] [PubMed] [Google Scholar]
- Stamps J. A., and Davis J. M.. . 2006. Adaptive effects of natal experience on habitat selection by dispersers. Anim. Behav. 72: 1279–1289. [Google Scholar]
- Steidle J. L. M. 1998. Learning pays off: influence of experience on host finding and parasitism in Lariophagus distinguendus. Ecol. Entomol. 23: 451–456. [Google Scholar]
- Stephens D. W. 1993. Learning and behavioral ecology: incomplete information and environmental predictability, pp. 195–218. InPapaj D. R. and Lewis A. C. (eds.), Insect learning: ecology and evolutionary perspectives. Springer US, Boston, MA. [Google Scholar]
- Stevens T. J. III, Kilmer R. L., and Glenn S. J.. . 2000. An economic comparison of biological and conventional control strategies for whiteflies (Homoptera: Aleyrodidae) in greenhouse poinsettias. J. Econ. Entomol. 93: 623–629. [DOI] [PubMed] [Google Scholar]
- Stopfer M., and Laurent G.. . 1999. Short-term memory in olfactory network dynamics. Nature. 402: 664–668. [DOI] [PubMed] [Google Scholar]
- Storeck A., Poppy G. M., Emden H. F., and Powell W.. . 2000. The role of plant chemical cues in determining host preference in the generalist aphid parasitoid Aphidius colemani. Entomol. Exp. Appl. 97: 41–46. [Google Scholar]
- Strausfeld N. J., Hansen L., Li Y., Gomez R. S., and Ito K.. 1998. Evolution, discovery, and interpretations of arthropod mushroom bodies. Learn. Mem. 5: 11–37. [PMC free article] [PubMed] [Google Scholar]
- Suckling D. M., and Sforza R. F.. . 2014. What magnitude are observed non-target impacts from weed biocontrol? PLoS One 9: e84847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto H., Powell W., Pickett J., Kainoh Y., and Takabayashi J.. . 2009. Learning is involved in the response of parasitic wasps Aphidius ervi (Haliday) (Hymenoptera: Braconidae) to volatiles from a broad bean plant, Vicia faba (Fabaceae), infested by aphids Acyrthosiphon pisum (Harris) (Homoptera: Aphididae). Appl. Entomol. Zool. 44: 23–28. [Google Scholar]
- Tauber M. J., Tauber C. A., Daane K. M., and Hagen K. S.. . 2000. Commercialization of predators: recent lessons from green lacewings (Neuroptera: Chrysopidae: Chrosoperla). Am. Entomol. 46: 26–38. [Google Scholar]
- Teasdale J. R., Abdul-Baki A. A., Mill D. J., and Thorpe K. W.. . 2004. Enhanced pest management with cover crop mulches. Acta Hort. 638: 135–140. [Google Scholar]
- Thompson J. N. 1988. Evolutionary ecology of the relationship between oviposition preference and performance of offspring in phytophagous insects. Entomol. Exp. Appl. 47: 3–14. [Google Scholar]
- Thorpe W. H. 1938. Further experiments on olfactory conditioning in a parasitic insect. The nature of the conditioning process. Proc Royal Soc. B. Biol. Sci. 126: 370–397. [Google Scholar]
- Thorpe W. H. 1939. Further studies on pre-imaginal olfactory conditioning in insects. Proc Royal Soc. B. Biol. Sci. 127: 424–433. [Google Scholar]
- Thorpe W. H., and Jones F. G. W.. . 1937. Olfactory conditioning in a parasitic insect and Its relation to the problem of host selection. Proc Royal Soc. B. Biol. Sci. 124: 56–81. [Google Scholar]
- Tison L., Holtz S., Adeoye A., Kalkan Ö., Irmisch N. S., Lehmann N., and Menzel R.. 2017. Effects of sublethal doses of thiacloprid and its formulation Calypso® on the learning and memory performance of honey bees. J. Exp. Biol. 220: 3695–3705. [DOI] [PubMed] [Google Scholar]
- Tully T., Cambiazo V., and Kruse L.. 1994. Memory through metamorphosis in normal and mutant Drosophila. J. Neurosci. 14: 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumlinson J. H., Lewis W. J., and Vet L. E. M.. . 1993. How parasitic wasps find their hosts. Sci. Am. 268: 100–106.8516669 [Google Scholar]
- Turlings T. C. J., Tumlinson J. H., Lewis W. J., and Vet L. E. M.. . 1989. Beneficial arthropod behavior mediated by airborne semiochemicals. VIII. Learning of host-related odors induced by a brief contact experience with host by-products in Cotesia marginiventris (Cresson), a generalist larval parasitoid. J. Insect Behav. 2: 217–225. [Google Scholar]
- Turlings T. C. L., Wäckers F. L., Vet L. E. M., Lewis W. J., and Tumlinson J. H.. . 1993. Learning of host-finding cues by hymenopterous parasitoids, pp. 51–78. InPapaj D. R., Lewis A. C. (eds.), Insect Learning. Springer US, Boston, MA. [Google Scholar]
- Ueno K., and Ueno T.. . 2005. Effect of wasp size, physiological state, and prior host experience on host-searching behavior in a parasitoid wasp (Hymenoptera: Ichneumonidae). J. Ethol. 23: 43–49. [Google Scholar]
- Veltman C. J., and Corbet S. A.. . 1991. In search of a model system for exploring the Chemical Legacy Hypothesis:Drosophila melanogaster and geraniol. J. Chem. Ecol. 17: 2459–2468. [DOI] [PubMed] [Google Scholar]
- Vergoz V., Roussel E., Sandoz J. C., and Giurfa M.. 2007. Aversive learning in honeybees revealed by the olfactory conditioning of the sting extension reflex. PLoS One 2: e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkerk R. H. J., Leather S. R., and Wright D. J.. . 1998. The potential for manipulating crop–pest–natural enemy interactions for improved insect pest management. Bull. Entomol. Res. 88: 493. [Google Scholar]
- Vet L. E. M., and Dicke M.. . 1992. Ecology of infochemical use by natural enemies in a tritrophic context. Ann. Rev. Entomol. 37: 141–172. [Google Scholar]
- Vet L. E. M., De jong AG, Franchi E., and Papaj D. R.. 1998. The effect of complete versus incomplete information on odour discrimination in a parasitic wasp. Anim. Behav. 55: 1271–1279. [DOI] [PubMed] [Google Scholar]
- Vet L. E. M., Lewis W. J., and Cardé R. T.. . 1995. Parasitoid foraging and learning, pp. 65–101. In Cardé R. T., Bell W. J. (eds.), Chemical Ecology of Insects 2 Springer US, Boston, MA. [Google Scholar]
- Vet L. E. M., Lewis W. J., Papaj D. R., and van Lenteren J. C.. . 1990. A variable-response model for parasitoid foraging behavior. J. Insect Behav. 3: 471–490. [Google Scholar]
- Vet L. E. M., and Van Opzeeland K.. . 1984. Olfactory microhabitat selection in Leptopilina heterotoma (Thomson) (Hym.: Eucoilidae), a parasitoid of Drosophilidae. Neth. J. Zool. 35: 497–504. [Google Scholar]
- Vinauger C., Buratti L., and Lazzari C. R.. 2011a. Learning the way to blood: first evidence of dual olfactory conditioning in a blood-sucking insect, Rhodnius prolixus. I. Appetitive learning. J. Exp. Biol. 214: 3032–3038. [DOI] [PubMed] [Google Scholar]
- Vinauger C., Buratti L., and Lazzari C. R.. 2011b. Learning the way to blood: first evidence of dual olfactory conditioning in a blood-sucking insect, Rhodnius prolixus. II. Aversive learning. J. Exp. Biol. 214: 3039–3045. [DOI] [PubMed] [Google Scholar]
- Vinauger C., Lutz E. K., and Riffell J. A.. 2014. Olfactory learning and memory in the disease vector mosquito Aedes aegypti. J. Exp. Biol. 217: 2321–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wäckers F. L., and Lewis W. J.. . 1994. Olfactory and visual learning and their combined influence on host site location by the parasitoid Microplitis croceipes (Cresson). Biol. Control 4: 105–112. [Google Scholar]
- Walsh B. D. 1864. On phytophagic varieties and phytophagic species. Proc. Entomol. Soc. Philadelphia. III: 403–430. [Google Scholar]
- Wäschke N., Meiners T., and Rostás M.. . 2013. Foraging strategies of parasitoids in complex chemical environments, pp. 37–63. InWajnberg E. and Colazza S. (eds.), Chemical ecology of insect parasitoids. John Wiley & Sons, Ltd, Chichester, United Kingdom. [Google Scholar]
- Weiss M. R., Wilson E. E., and Castellanos I.. . 2004. Predatory wasps learn to overcome the shelter defences of their larval prey. Anim. Behav. 68: 45–54. [Google Scholar]
- Wisenden B. D., Chivers D. P., and Smith R. J. F.. . 1997. Learned recognition of predation risk by Enallagma damselfly larvae (Odonata, Zygoptera) on the basis of chemical cues. J. Chem. Ecol. 23: 137–151. [Google Scholar]
- Yang Z., Wei J., and Wang X.. . 2006. Mass rearing and augmentative releases of the native parasitoid Chouioia cunea for biological control of the introduced fall webworm Hyphantria cunea in China. Biocontrol. 51: 401–418. [Google Scholar]