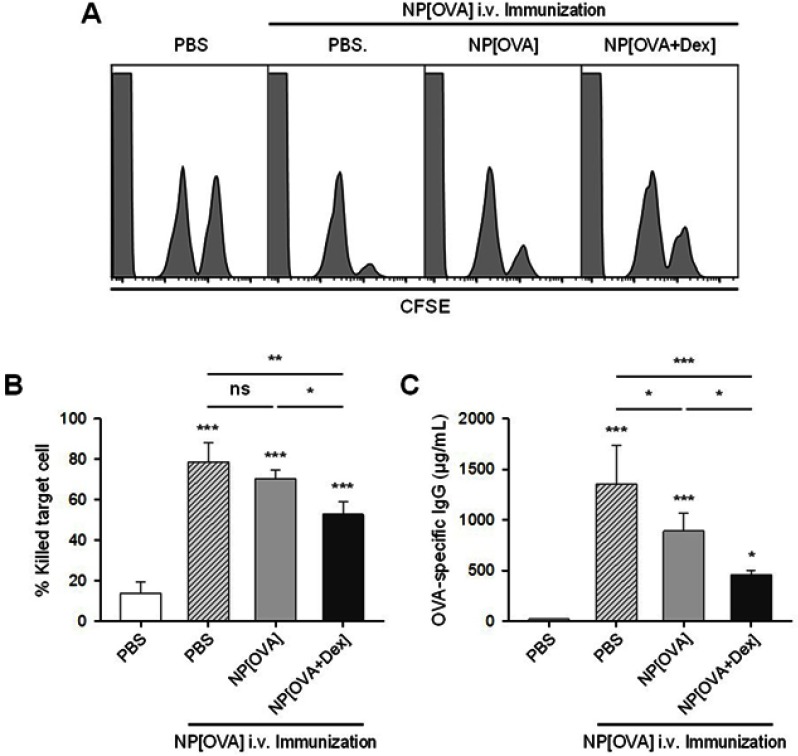
Figure 6.
Effect of oral treatment of NP[OVA+Dex] on OVA-specific responses in vivo.
Notes: (A) Induction of OVA-specific CTLs. PBS, NP[OVA], or NP[OVA+Dex] (200 μg/mouse as OVA) was i.g. injected into C57BL/6 mice on day 0 and 2. On day 9, mice were i.v. injected with NP[OVA] (80 μg/mouse as OVA) or PBS. After 7 days, OVA-specific cytotoxic activity was assessed using an in vivo CTL assay, as described in Figure 5A. (B) The proportion of killed target cells in each experimental group is shown. The data are presented as the mean ± SD of three independent experiments. (C) Production of OVA-specific IgG. PBS, NP[OVA], or NP[OVA+Dex] (200 μg/mouse as OVA) was i.g. injected into C57BL/6 mice on day 0 and 2. On day 9, mice were i.v. injected with NP[OVA] (80 μg/mouse as OVA), or PBS. After 7 days, the sera were collected from the mice, and OVA-specific IgG levels were measured by ELISA. The data are presented as the mean ± SD of three independent experiments. *P<0.05, **P<0.01, ***P<0.001. “ns” indicates no significant difference.
Abbreviations: CTL, cytotoxic T lymphocyte; Dex, dexamethasone; ELISA, enzyme-linked immunosorbent assay; i.g., Intragastric; IgG, immunoglobulin G; i.v., intravenous; NP[OVA+Dex], nanoparticles containing ovalbumin and dexamethasone; NP[OVA], nanoparticles containing only ovalbumin; OVA, ovalbumin; PBS, phosphate-buffered saline; SD, standard deviation.