Fig. 1 |. The anatomy and physiology of respiration.
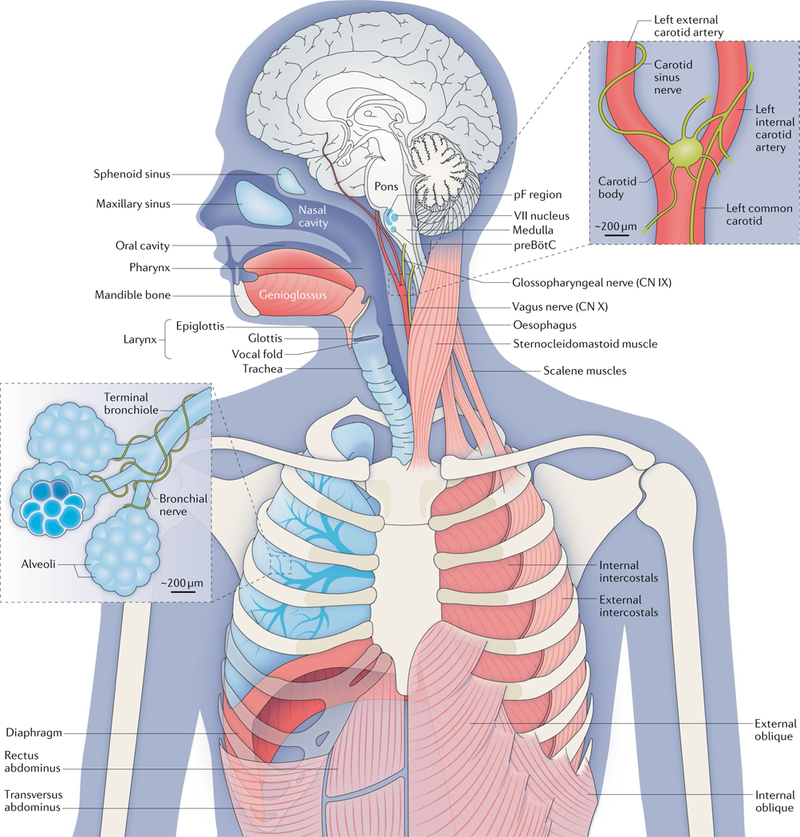
Breathing movements depend on pump, resistance and accessory muscles. Pump muscles include the dome-like, uniquely mammalian diaphragm and the external intercostals. During inspiration, descent of the diaphragm combined with rib elevation by the external intercostals expands the lungs to draw in air (assuming the airways are patent). The oblique abdominals and transversus abdominus and internal intercostals are expiratory pump muscles. Airway resistance muscles, which modulate inspiratory and expiratory airflow, include skeletal muscles of the tongue (including the genioglossus (shown) and the hyoglossus, styloglossus and stylohyoid muscles (not shown)), glottis, larynx and pharynx, as well as smooth muscle of the bronchi. The sternocleidomastoid and scalene muscles are accessory muscles that stabilize the rib cage. The gas-exchanging surface of human lungs, consisting of ~5 × 108 alveoli each measuring 200 μm in diameter, is roughly half the size of a tennis court (~70 m2) but is contained in a volume of <3 litres. At rest, we inhale and exhale ~5 litres of air per minute (~10 × 500 millilitre breaths per minute, containing ~ 1 litre of O2); we extract from the inspired air ~250 millilitres of O2 per minute to support metabolism and add to the expired air ~200 millilitres of CO2 per minute. The regulation of breathing relies on feedback from peripheral and central chemosensors. Carotid bodies, at the branch point of the carotid arteries, monitor the partial pressure of O2 (pO2), the partial pressure of CO2 (pCO2) and pH in arterial blood and signal to the brainstem via the glossopharyngeal nerve (cranial nerve (CN) IX). In the brainstem, chemosensory neurons and glia in the ventral parafacial nucleus (pFV) and other regions detect and respond to fluctuations in CO2 levels and pH in the cerebrospinal fluid. These neurons project paucisynaptically to the preBötzinger Complex (preBötC) and other sites to influence breathing to maintain homeostasis. Breathing in mammals (and other air breathers) under most conditions is extremely sensitive to changes in CO2 levels that directly affect pH. For example, in resting humans, an ~2.5% increase in pCO2 from 40 to 41 mmHg will increase ventilation ~40% from ~5 to ~7 litres per minute. By contrast, breathing is relatively insensitive to changes in O2 levels at rest. The O2–haemog1obin dissociation curve is fairly flat at normal levels of arterial O2 (100 ± 20 mm Hg), meaning that haemoglobin is >96% saturated with O2 even if breathing increases substantially. However, under certain conditions (for example, high altitude or intense exercise), hypoxia provides a powerful stimulus to breathe. Continuous breathing comes at a considerable metabolic cost insofar as the respiratory muscles are the only skeletal muscles that are active during all sleep and wake states. To counter this, mammals evolved a powerful diaphragm that is sufficient to inflate the lungs, and a resting breathing pattern in which inspiration (and usually postinspiration) is active (requiring muscle contraction), whereas expiration is passive. In birds (and most lower vertebrates) that do not have a diaphragm, inspiration and expiration are both active at rest. Central breathing networks are also modulated by mechanosensory feedback regarding the status of the pump muscles and lungs. Stretch- receptor afferents in airway smooth muscle encode volume-related information via the vagus nerve (CN X), which is crucial in maintaining optimal lung volumes for efficient breathing. This feedback also underlies Breuer-Hering reflexes, which are important in controlling the timing and pattern of each breath. Lung stretch-receptor afferents and their central relay interneurons rhythmically inhibit the inspiratory preBötC and excite the expiratory lateral parafacial nucleus (pFL) when lungs are inflated (inspiratory termination reflex) and conversely excite the preBötC and inhibit the pFL when lungs are deflated. VII nucleus, facial motor nucleus. The figure is based on a drawing contributed by J. Milstein.
