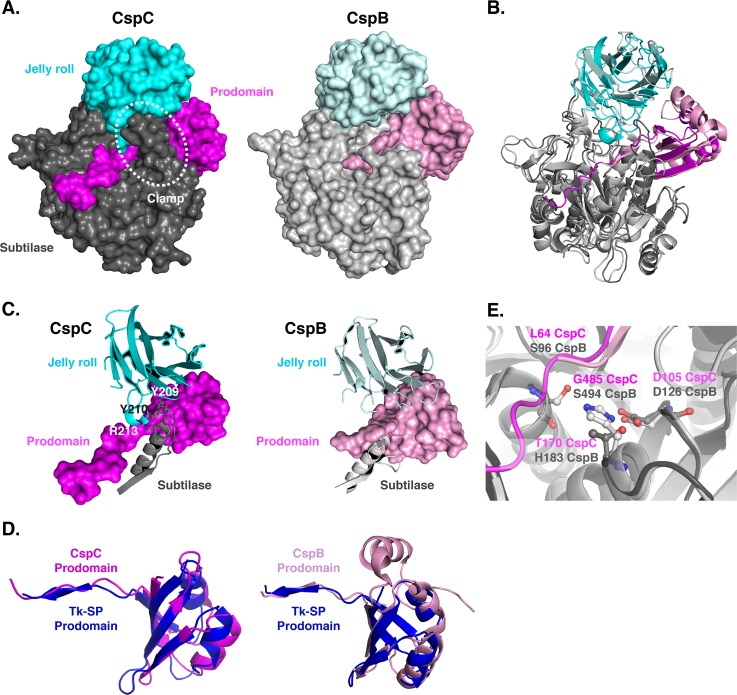
Fig 1. Structural comparison of C. difficile CspC to C. perfringens CspB.
(A) Space-fill models of CspC and CspB [32] with the respective prodomains colored in pink, subtilase domains in grey, and jell roll domains in cyan (lighter tones denote CspB). The prodomain “clamp” in CspC formed by interactions between the jelly roll domain and subtilase domains is highlighted. (B) Ribbon representation overlay of CspC and CspB structures. A least squares superposition of the subtilase domain reveals a 1.03 Å rms over 1200 atoms of the backbone trace. (C) Comparison of the CspC prodomain “clamp” relative to CspB. The jelly roll domain interacts with a clamping loop from the CspC subtilase domain through which the CspC prodomain is threaded. Specific residues involved in the CspC jelly roll-subtilase domain interactions are marked. This interaction is absent in CspB due to a shorter subtilase domain loop, which is in a different orientation, and the disorder of the jelly roll domain interacting loop. (D) Superposition of the CspC and CspB prodomains, respectively, with the canonical prodomain from Tk-subtilisin [36] (blue). (E) Overlay of the CspB catalytic triad (S494, H183 and D126) to CspC (G485, T170 and D105). The position of the terminal residue of the CspB prodomain (S96) as well as the equivalent residue in CspC’s uncleaved prodomain (L64) is indicated.