Abstract
Work during the past two decades has highlighted how HIV contributes to hepatic inflammation and fibrosis, leading to changes in the timing of antiretroviral therapy initiation and to improved diagnosis and management of liver disease in patients with HIV. As this population ages, clinician vigilance with early detection of emerging liver disease will be critical.
During the past decade, our understanding of chronic liver disease in patients with HIV has tremendously expanded. New insights changed recommendations of when to initiate antiretroviral therapy (ART) and have improved diagnosis and management of liver disease in this population. In this Comment, we outline mechanisms by which HIV contributes to hepatic inflammation and fibrosis and discuss effects of HIV infection on the spectrum and prognosis of liver diseases to highlight how this understanding affects clinical management. Co-infection with HCV or HBV in patients with HIV is common; thus, most information on the effect of HIV on liver fibrosis progression is derived from co-infected individuals. These patients have a higher relative risk (RR) of cirrhosis, increased development of decompensated cirrhosis and accelerated fibrosis progression rates compared with those who are only infected with HCV or HBV1,2. Furthermore, rapid fibrosis correlates with reduced CD4+ T cell counts and detectable plasma HIV levels.
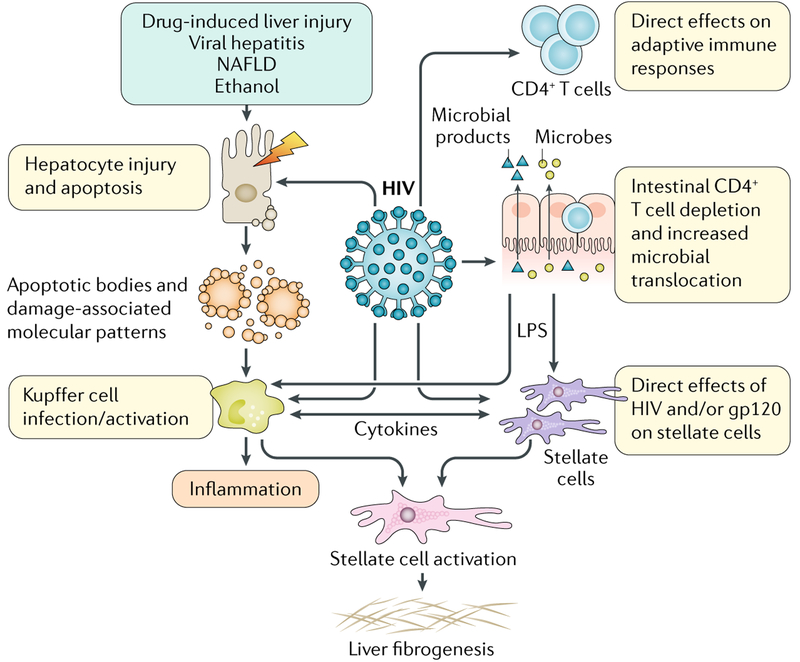
With HIV infection, multiple pathways converge on activated hepatic stellate cells (HSCs), the primary source of collagen in the injured liver, promoting hepatic inflammation and fibrosis (FIG. 1). First, HIV-induced depletion of CD4+ T cells relative to CD8+ T cells alters the hepatic cytokine profile, establishing a fibrogenic environment. HIV and/or its envelope protein, gp120, promote direct pro-fibrogenic effects on HSCs, secretion of pro-inflammatory cytokines (such as MCP1) and hepatocyte apoptosis3. In vitro, HIV can infect HSCs, sinusoidal endothelial cells and Kupffer cells, the resident macrophages of the liver, but evidence of in vivo infection has been demonstrated most consistently for Kupffer cells. These interactions are relevant for highly viraemic patients, but whether they remain relevant in patients whose infection is controlled by ART is a key question. In the D:A:D study, which included patients whose HIV infection was well-controlled by ART, liver-related death was the leading cause of non-AIDS death (adjusted RR of liver death 1.67, even with CD4+ T cell counts <350 cells/mm3)4. Together with data that associate ART use with reduced rates of hepatic fibrosis in patients with HCV or HBV coinfection and increased understanding of direct effects of HIV on fibrogenesis, these findings led to the recommendation that ART should be started irrespective of CD4+ T cell counts or at least when counts are <500 cells/mm3 in patients with concomitant viral hepatitis. On the basis of results from studies like the START trial, ART initiation is now recommended irrespective of CD4+ T cell counts in all HIV-infected patients5. Hence, monitoring for drug-induced liver injury is important. Acute liver injury is unpredictable but easily recognized shortly after drug initiation. Chronic liver injury, such as non-cirrhotic portal hyper-tension, is more variable and was observed with drugs such as didanosine. The introduction of less hepatotoxic drugs has reduced the occurrence of chronic injury but vigilance remains important.
Fig. 1 |. Promotion of hepatic inflammation and fibrosis by HIV in chronic liver disease.
LPS, lipopolysaccharide; NAFLD, nonalcoholic fatty liver disease. Image courtesy of Jill Gregory, Icahn School of Medicine at Mount Sinai.
Early initiation of ART and advances in the treatment of viral hepatitis have greatly improved liver outcomes in patients with HIV, but some HIV-related alterations are not restored by ART and contribute to poor outcomes despite ART. In particular, persistent CD4+ T cell depletion in the gut and increased gut permeability might be critical factors in promoting microbial translocation and immune activation. Plasma lipopolysaccharide (LPS) levels decrease in patients treated with ART, but they never return to the levels of individuals without HIV6. In addition, microbial translocation is associated with persistent macrophage activation despite viral suppression or reconstitution of CD4+ T cell levels7. Kupffer cells are the first line of defence against gut-derived pathogens, responsible for clearing translocated products. Although normally immunotolerant, HIV-1-infected Kupffer cells have an exaggerated pro-inflammatory and pro-fibrogenic response to LPS8. Unpublished data from our own group suggest that despite undetectable viral loads, Kupffer cells derived from patients with HIV continue to hyperrespond to LPS. The importance of the gut–liver axis in driving fibrosis progression in several liver diseases has been postulated9, and it is compelling to think about additional effects of HIV in altering the gut microbiome, directly promoting microbial trans-location, accelerating fibrosis progression and chronic immune activation.
Viral hepatitis in patients with HIV is now easily treated, new ARTs with fewer direct hepatotoxic effects are started much earlier and patients live longer. Thus, the spectrum of liver disease in this population is evolving and chronic liver diseases, such as nonalcoholic fatty liver disease (NAFLD), are becoming more frequent. The prevalence of NAFLD in patients who are HIV mono-infected ranges from 30% to 60%10. Furthermore, given the increased ease of obtaining sustained virological response in patients with HCV infection, both unmasked underlying concomitant NAFLD and de novo fatty liver disease are now being observed. Hepatic steatosis in those who are infected by HIV only can be induced by direct effects of HIV on lipogenesis, by metabolic dysfunction related to HIV or long-term ART or by HIV-related chronic inflammation. ART, especially protease inhibitors, can alter hepatic free fatty acid composition by inducing insulin resistance and dyslipidaemia10.
As our understanding of the effects of HIV on the liver is increasing, attention turns to optimized management. Early initiation of ART and well-tolerated effective treatments for viral hepatitis have informed clear strategies to improve liver disease outcomes in all patients with HIV. This includes universal screening for HBV and HCV infection and treatment of HIV–HCV and HIV–HBV co-infected patients as early as possible with both ART and agents for HCV or HBV, respectively. Although no clear guidelines for liver cancer screening exist specifically for patients with HIV, on the basis of recommendations for HCV or HBV mono-infected patients, liver cancer screening every 6 months with ultrasonography is suggested for patients with HIV and HCV with advanced liver disease (stage >3 fibrosis) and for those with HIV and HBV irrespective of the fibrosis stage.
Screening for metabolic complications and fatty liver in patients with HIV should become standard of care. Providers need to recognize that any elevation in liver enzymes must be evaluated. Fibrosis is the single greatest predictor for nonalcoholic steatohepatitis (NASH) progression; thus, non-invasive assessments (serum and liver elastography with controlled attenuation parameter) can help differentiate patients with simple steatosis from those with fibrosis, thereby stratifying those in need of confirmatory liver biopsy. Lifestyle modification, weight loss and control of metabolic syndrome, if present, are needed in all patients with NAFLD/NASH. Although no treatments for NASH are currently FDA-approved (even in the general population), numerous clinical trials are underway and we anticipate inclusion of patients with HIV in trials in the next two years.
As patients with HIV infection become older and our understanding of the unique features of liver disease in these patients increases, early detection of liver disease is the key to improved clinical outcomes.
Acknowledgements
M.B.B. is supported by the NIH grant 5R01DK108364.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Benhamou Y et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology 30, 1054–1058 (1999). [DOI] [PubMed] [Google Scholar]
- 2.Puoti M et al. Natural history of chronic hepatitis B in co-infected patients. J. Hepatol 44, S65–S70 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Tuyama AC et al. Human immunodeficiency virus (HIV)-1 infects human hepatic stellate cells and promotes collagen I and monocyte chemoattractant protein-1 expression: implications for the pathogenesis of HIV/hepatitis C virus-induced liver fibrosis. Hepatology 52, 612–622 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber R et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch. Intern. Med 166, 1632–1641 (2006). [DOI] [PubMed] [Google Scholar]
- 5.INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N. Engl. J. Med 373, 795–807 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenchley JM et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med 12, 1365–1371 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Wallet MA et al. Microbial translocation induces persistent macrophage activation unrelated to HIV-1 levels or T cell activation following therapy. AIDS 24, 1281–1290 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosoian A et al. Frontline science: HIV infection of Kupffer cells results in an amplified proinflammatory response to LPS. J. Leukoc. Biol 101, 1083–1090 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balagopal A et al. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology 135, 226–233 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lemoine M, Serfaty L & Capeau J From nonalcoholic fatty liver to nonalcoholic steatohepatitis and cirrhosis in HIV-infected patients: diagnosis and management. Curr. Opin. Infect. Dis 25, 10–16 (2012). [DOI] [PubMed] [Google Scholar]