Abstract
This overview on skin delivery considers the evolution of the principles of percutaneous ab-sorption and skin products from ancient times to today. Over the ages, it has been recognised that products may be applied to the skin for either local or systemic effects. As our understanding of the anatomy and physiology of the skin has improved, this has facilitated the development of technologies to effectively and quantitatively deliver solutes across this barrier to specific target sites in the skin and beyond. We focus on these technologies and their role in skin delivery today and in the future.
Keywords: History, skin delivery, nanotechnology, physical enhancement, microneedles, minimally invasive, transdermal technologies, targeted delivery, stratum corneum, follicular transport, wearable devices
1. SKIN DELIVERY PAST
Ointments and potions made of animal, mineral or plant extracts were in common use in ancient Egyptian and Babylonian medicine in 3000 BC [1]. For example, a Sumerian clay tablet dates back to 2100 BC, describing a formulation made of pulverised snake and bat’s dung incorporated into an aqueous paste of plant extracts and earths for the treatment of skin disease [2]. Topical remedies that were anointed, bandaged, rubbed or applied to the skin for the treatment of skin conditions in ancient Egypt are detailed in the Ebers Papyrus, dating from 1550 BC [3]. The Greek physician Claudius Galenus (commonly known as Galen, 129-199 AD), introduced the compounding of herbal drugs to Western medicine and developed a formula for a cold cream (Galen’s Cerate) which was similar to those available today. The Persian physician Ibn Sina (best known as Avicenna, 980-1037 AD), in his treatise The Canon of Medicine, proposed that topical drugs possess two spirits or states: the soft part that penetrates the skin and the hard part that does not [4]. Avicenna thus provided the first known mechanistic approach that underpins our current knowledge and pharmaceutical development in topical and transdermal delivery.
Our understanding of skin permeation has evolved from early observations of adverse systemic effects following skin exposure, such as systemic poisoning after belladonna plaster applications [5] and headaches after occupational exposure to nitroglycerin in explosives factories [6]. The observation that skin was relatively permeable to lipid-soluble substances but not to water and electrolytes [7] was explored in systematic in vitro studies conducted by Rein in the 1920s [8]. Moore et al. [9] showed that testosterone, testosterone propionate or estradiol applied to animal skin led to an enhancement in various hormonal responses. Zondek [10] described the cutaneous application of follicular hormone for amenorrhoea in 1938 and successful management of urogenital infections following topical application of an ointment containing the disinfectant chloroxylenol, in the early 1940s [11]. In 1948, a nitroglycerin ointment was successfully applied to treat Raynaud’s disease, by utilising its ability to permeate to and dilate the cutaneous blood vessels [12, 13]. Systematic studies identified that important determinants of skin permeability included drug solubility [14], partition coefficient and thermodynamic activity [15], drug-vehicle-skin interactions [16, 17], and skin temperature [18, 19]. In 1975, Michaels and co-workers [20] described a mathematical model of the stratum corneum and demonstrated that the skin diffusion coefficients of ten model drugs correlated with their water solubility and lipid-protein partition coefficient.
In the early 1970s, the Alza Corporation, through their founder Alejandro Zaffaroni, filed the first US patents describing transdermal delivery systems for scopolamine, nitroglycerin and nicotine. These delivery systems offered advantages including: (i) overcoming oral delivery limitations of vomiting and low bioavailability (e.g. due to a high liver and gut wall first-pass); (ii) providing an alternative to parenteral administration, which can be invasive, painful and risks bruising and infection; (iii) acting as a convenient, non-invasive means to achieve relatively constant and reliable blood levels over 24 to 72 hrs, whilst also being able to cease the delivery at any time by removing the patch [21].
2. SKIN DELIVERY PRESENT
In addition to the range of drugs applied for local effects in the skin and associated tissues, nearly twenty drugs have been successfully developed for transdermal delivery using various topical dosage forms such as patches, gels and ointments and cutaneous solutions [21]. Four drugs using transdermal patches for the management of central nervous system disorders were approved in a two-year period: methylphenidate (Daytrana®, 2006), selegiline (Emsam®, 2006), rivastigmine (Exelon®, 2007) and rotigotine (Neupro®, 2007) for the management of attention deficit hyperactivity disorder, depression, Alzheimer’s disease and Parkinson’s disease, respectively [22]. Interestingly the rotigotine patch is the first patch that has been originally developed for transdermal delivery while other drugs now delivered through the skin have previously been administered using more conventional oral and parenteral dosage forms [23]. Fig. (1) summarizes the key transdermal drugs used today and their recommended application sites [21]. Continued development for topical and transdermal delivery is providing new technologies for controlled dosing, targeted site-specific delivery, and/or enhanced skin penetration, thereby extending the range of therapeutic compounds that can be applied via the skin.
Fig. (1).
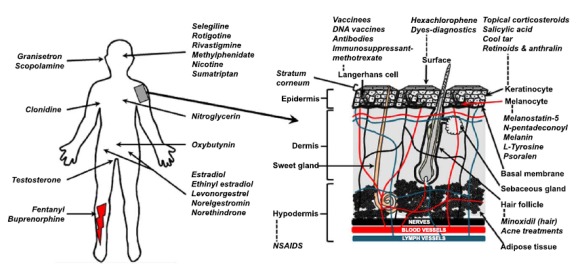
A. Approved and underdevelopment transdermal drugs and their recommended site of application. B. Close-up of human skin and various sites and targets within the skin.
3. SKIN AS A TARGET SITE
The skin is a complex multilayered structure (Fig. 2) extending over an area of 1.5 to 2 m2 in adults. Its primary role is to act as a barrier between the body and the relatively hostile external environment. The stratum corneum is a highly structured lipid-rich region that minimises the ingress and egress of water, oxygen and chemicals. Topically applied products may target sites in one or more different skin layers (i.e. epidermis, dermis and hypodermis), the skin appendages (e.g. hair follicles with associated sebaceous glands, sweat glands and nails), and underlying tissues. The key target region for the majority of topical products is the viable epidermis and sites targeted here include nerves, keratinocytes, melanocytes, Langerhans cells and hair follicles (Fig. 2). Transdermal products target the systemic circulation.
Fig. (2).
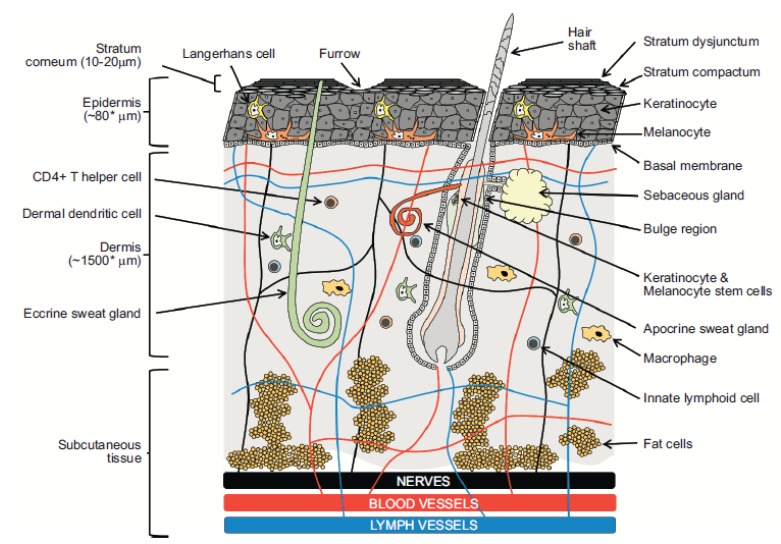
Diagrammatic illustration of human skin. Barriers, sites of action and drug delivery opportunities.
Most solutes suitable for transdermal delivery are characterized by low molecular weight, low melting point, moderate lipophilicity, and few hydrogen bonding sites. Table 1 summarizes the properties of current transdermally delivered drugs.
Table 1. Physicochemical, pharmacokinetic properties and indications of currently marketed transdermal drugs.
| Drug | Dose/day (mg) | MW (Da) |
MP
(°C) |
Log P | Saq (mg/mL) | Clearance (cl) L.h-1 | t1/2 (h) | Oral F (%) | Plasma Concentration | Estimated Jskin Required (µg/h) | In vivo J skin (µg/cm2/h) | Product name | Indication |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buprenorphine | 0.12-1.68 | 468 | 209 | 3.8 | 0.047, 0.008 (32°C) | 77 i.v. 55 i.m. |
22-36 | 51 sI 28 bc |
0.1-0.4 | 7.7 | 0.8 | Butrans | Moderate to severe pain |
| Clonidine | 0.1-0.3 | 230 | 130 | 2.7 | 0.17, 13.58 | 15 | 6-20 | 95 | 0.2-2.0 | 3-30 | 1.2 | Catapres- TTS |
Hypertension |
| Ethinyl oestradiol | - | 296 | 141-146 | 4.3 | 0.039, 0.0092 (25°C) | 70 | 7.7 p.o, 17 t.d. | 55 | 0.025-0.075 | 1.75-5.25 | 0.07 | Ortho Evra | Female contraception |
| Fentanyl | 0.288-2.400 | 337 | 83-84 | 3.9 | 0.15, 0.2 (30°C), 0.2 (25°C) |
27-75 | 3-12 | 50 | 1-3 | 27-225 | 2.4 | Duragesic | Chronic pain |
| Granisetron | 3.1 | 312 | 152-154 | 2.6 | 0.017 | 33-76 | 4-6 | 60 | 3.9 (t.d. Mean C max) |
129-296 | 2.5 | Sancuso | Chemotherapy-induced emesis |
| Glyceryl trinitrate | 2.4-15.0 | 227 | 13, liquid | 1 | 0.66, 1.3 (30°C) | 216-3270 | 0.03-0.05 | < 1 | 0.1-5 | 4.32-1308 | 20,30 | Nitro-Dur | Angina |
| Estradiol and Levonorgesterel | 0.050, 0.0007-0.015 | 272, 312 | 173-179 235-237 |
4.2 3.8 |
0.003 (30°C) 0.0015 (25°C) 0.017 |
600-800 5.7 |
Approx. 1p.o. 28t.d19.3p.o. |
3-5 | 0.03-0.05 0.1-0.2 |
24-48 1 |
0.63, 0.09, 0.12, 0.18, 0.14, 0.23, 0.42, 0.17 0.2 0.03 |
Climara Pro | Female contraception |
| Methylphenidate | 26.0 - 80.0 | 233 | 74-75, liquid | 2.1 | 1.8 | 12(d); 21(1) (children 30kg) | 1.5-5 | 5-20 | 5-25 | 60-315 | 88 | Daytrana | ADHD |
| Nicotine | 7.0-21.0 | 162 | -79, liquid | 1.1 | 62,1085 (30°C) | 77 | 2 | 20-45 | 5-30 | 385-2310 | 29, 69, 31, 40 | Nicoderm | Smoking cessation |
| Norelgestromin | 0.2 | 327 | 110-130 | 3.67 | 0.0043 | - | 28 | - | 0.6-1.2 | - | 0.31 | Ortho Evra | Female contraception |
| Norethindrone acetate | 0.125-0.250 | 341 | 161-162 | 3.2 | 0.0065 | 20.6 | 6-8 | 60 | 0.5-0.8 | 10.3-16.5 | 0.65 | Combipatch | Female HRT |
| Oxybutynin | 3.9 | 358 | 56-58 | 4.3 | 10-64 | 2 | 6 | 0.5-5 | 5-192 | 4.2 | Oxytrol | Enuresis | |
| Oestradiol | 0.025-0.100 | 272 | 173-179 | 4.2 | 0.003 (30°C) 0.0015 (25°C) |
600-800 | 0.05 | 3-5 | 0.04-0.06 | 24-48 | 0.2,0.17,0.14, 0.12,0.42,0.18, 0.63,0.23, 0.09 |
VivelleDot | Female HRT |
| Rivastigmine | 4.6-9.5 | 250 | Oil at 25°C | 2.3 | 25 | 108 | 1.5 | 36 | 2.5-20 | 270-2160 | 39 | Exelon | Alzheimer’s disease |
| Rotigotine | 1.0-3.0 | 316 | 75-77 | 4.7 | 0.017 | 600 | 5-7 | - | 0.4-2 | 240-1200 | 8.3 | Neupro | Parkinson’s disease & restless leg syndrome |
| Scopolamine | 0.3 | 303 | 55, liquid | 0.8 | 1.8, 75 (30°C) | 65-121 | 1-5 | 4-27 | 0.04 | 3.25-6.05 | 5.6 | Transderm Scop | Travel sickness |
| Seligiline | 6.0-12.0 | 187 | Liquid at 25°C | 2.7 | 0.73 | 84 | 10 | 4,10 | 2 | 168 | 12.5 | Emsam | Depression |
| Testosterone | 0.3-5.0 | 288 | 155 | 3.6 | 0.02 | 41 | 0.17-1.7 | <1, 7 | 3-10.5 | 123-430.5 | 13.9 | Androderm | Hypogonadism |
Abbreviation: i.v. intravenous, i.m. intramuscular, p.o. per oral, t.d. transdermal, s.I. sublingual, bc buccal.
The time course of the drugs in the body is defined by the flux of the solute’s entry into the skin, and for local effects, clearance from the skin, or for systemic effects, clearance from the systemic circulation. Steady state transport flux (J) across a plane sheet membrane of area A and thickness h with a constant diffusivity D, and diffusion path length h can be described in terms of the interior membrane concentrations; C, at the interior interface adjacent to the product and 0, adjacent to a sink receptor [24]. Applying this to transport across the stratum corneum membrane, and assuming that the regions below that major diffusion barrier offer essentially sink conditions, the steady-state flux can be described by the expression:

Non-sink conditions can slow down skin permeation as well as increase local skin concentrations of solutes [25].
The maximum, or saturated flux Jmax, in turn, relates to the solubility of the solute in the stratum corneum (Ssc):

4. SKIN DELIVERY TECHNOLOGIES FOR PENETRATION ENHANCEMENT AND SITE-SPECIFIC TARGETING
The primary challenge to optimising skin delivery and extending the range of compounds that can be effectively delivered via the skin is overcoming the barrier properties of the skin. The stratum corneum is an effective barrier that limits skin penetration and consequently the extent to which the skin can be effectively used to deliver compounds for therapeutic outcomes in the skin, underlying tissues or systemically. Many compounds do not possess the physicochemical criteria [i.e. low molecular weight, adequate lipophilicity (log Po/w ≅ 1-3) and low melting point] to passively permeate the skin in therapeutic quantities thus limiting the topical and transdermal market. Development of technologies to enhance delivery into the skin has been a major research focus for over half a century. ‘Passive’ technologies involve the use of formulation excipients and chemical penetration enhancers, and various types of micro and nano-delivery systems [26-28]. Technologies using an external driving force (‘active’ or ‘physical’ enhancement methods) have employed electrical (iontophoresis and electroporation), thermal (laser and radiofrequency thermal ablation), ultrasound, mechanical (microneedles), and velocity (jet injector) based approaches [29].
5. COLLOIDAL NANOSYSTEMS
Colloidal systems used in skin delivery are typically lipid-based (Fig. 3) and include nanoemulsions (NE), liposomes or flexible vesicles, nanostructured lipid carriers (NLC) and solid lipid nanoparticles (SLN). These nanosystems can offer the potential to improve bioavailability and efficacy, target delivery to skin regions and follicles, increase the stability of active, and facilitate the formulation of lipophilic, poorly water-soluble compounds. They may also have a role in combination with other penetration enhancement technologies.
Fig. (3).
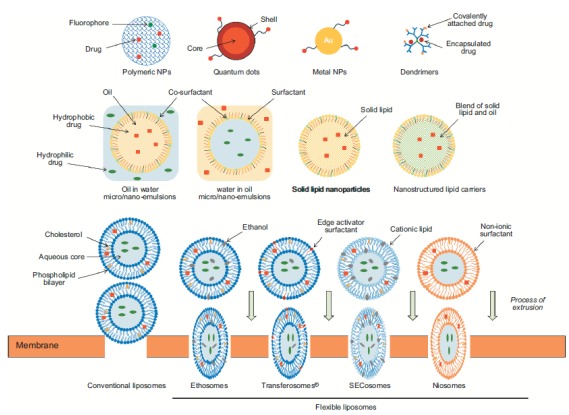
Nanodelivery systems for topical and transdermal drug delivery. (Adapted with permission from Roberts et al. 2016 [26]).
5.1. Nanoemulsions (NE) and Microemulsions (ME)
Are transparent, monophasic, optically isotropic colloidal dispersions composed of oil, water, surfactant and cosurfactant with droplet sizes less than 100 nm and a low polydispersity [27]. ME are thermodynamically stable whilst NE are kinetically stable, with both providing a large surface area and uniform distribution on the skin. The primary difference between NE and ME is their thermodynamic stability [30], which also results in the higher energy required to form NE compared to ME. NE typically contain a relatively low surfactant content with reduced irritancy to the skin. They enhance permeation into the skin by: (i) their high solubilization capacity for both lipophilic and hydrophilic compounds increase the loading capacity and dose application; (ii) their large surface area and good skin contact, coupled with their occlusive nature ensures surface contact with the stratum corneum; (iii) their oil and surfactant components may have a direct permeation enhancement effect on the stratum corneum lipid structure. We showed significantly enhanced flux of caffeine and naproxen across human epidermis in vitro applied as a NE containing the skin penetration enhancers oleic acid or eucalyptol [31]. The NE provided a modest increase in solubility within the stratum corneum of hydrophilic caffeine, therefore the increase in flux was due primarily to the direct effect of the NE excipients reducing the diffusional resistance for caffeine penetration within the stratum corneum. In contrast, increased flux of the lipophilic naproxen was primarily due to NE increased solubility of naproxen in the stratum corneum, with only a modest increase in diffusivity.
5.2. Solid Lipid Nanoparticles (SLN)
Are composed of lipids that are solid at room temperature, that are stabilised as a nanodispersion by a surface covering of surfactant [32]. They facilitate the formulation and increase the stability of lipophilic compounds such as retinol that are prone to decomposition in the presence of light and oxygen [33], increase the deposition in the stratum corneum and reduce flux [34]. Enhanced stratum corneum permeation is attributed to: (i) prolonged contact with the skin surface; (ii) their occlusive nature due to formation of a film on the skin surface that combines with the skin lipid film to reduce water loss thus hydrating the skin [35]; and (iii) interaction between formulation lipids and stratum corneum lipids facilitating permeation of lipid-soluble compounds [36]. Their primary applications are for skin targeting such as that demonstrated by curcumin SLNs which showed controlled release over 24 hrs, and effective deposition for the reduction of skin pigmentation and inflammation in Balb/c mouse skin [37]. The use of cationic lipids offers the potential for charge interaction with the negatively charged skin surface. For example, highly positively charged (51 mV) SLN using the cationic phospholipids 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) and dioleoylphosphatidylethanolamine (DOPE), Tween 20 as surfactant, tricaprin as a solid lipid core, and encapsulating plasma DNA, showed enhanced in vitro permeation into mouse skin and expression of mRNA in vivo in mice after topical application [38].
5.3. Nanostructured Lipid Carriers (NLC)
Are composed of a fluid lipid phase embedded into a solid lipid matrix or localized at the surface of solid platelets and the surfactant layer [32]. The lipids spatial structure allows greater drug loading and better stability compared to SLN [39]. NLC and SLN are reported to have a similar mechanism of permeation enhancement, via occlusion and mixing between the formulation and stratum corneum lipids, however the liquid lipid component in NLC increases solubilization and thereby loading of the active, resulting in greater skin deposition [40, 41]. Surface modification with the cell-penetrating peptide transactivating transcriptional activator (TAT-NLC) showed greater epidermal deposition of a fluorescent probe and the lipophilic drug celecoxib, compared to control NLC, that were located mainly in the hair follicles following topical administration to rat skin in vitro [42]. They subsequently showed that surface modification of the NLC with a cell-penetrating peptide containing 11 arginines had significantly greater skin permeation enhancing ability compared to other polyarginines and TAT peptides [43].
5.4. Flexible Liposomes or Vesicles
Are composed of materials that will aggregate into bilayer structures to form spherical vesicles but have the ability to deform in shape. Various vesicle compositions have been developed, including compositions with materials known to have penetration enhancement properties including transfersomes (phospholipids with the surfactant sodium cholate) [44-47], ethosomes (phospholipids with a high proportion of ethanol) [48, 49], niosomes (flexible non-ionic surfactant vesicles) [50, 51], invasomes (phosphatidylcholine, ethanol and a mixture of terpene penetration enhancers) [52, 53], SECosomes (surfactant, ethanol and cholesterol) [54, 55], PEVs (penetration enhancer containing vesicles) [56-58]. Multiple mechanisms by which flexible vesicles enhance skin penetration have been suggested. First, that the vesicles disintegrate on the skin surface, their components penetrate into the stratum corneum, mixing with and modifying the lipid bilayers to indirectly enhance permeation [59, 60]. Cevc [61] suggested that intact flexible vesicles penetrate through the stratum corneum due to the transepidermal osmotic gradient, deforming to fit through ‘pores’ within the lipid bilayers. This hypothesis remains controversial, with recent evaluations of vesicle skin interactions using high-resolution technologies such as multiphoton excitation fluorescence microscopy imaging and cross correlation-raster image correlation spectroscopy (CC-RICS) [60] and stimulated emission depletion microscopy (STED) and CC-RICS [59] failing to show evidence of intact vesicles within the skin. STED provides image resolution to 20 nm, whilst CC-RICS allows tracking of two fluorescent probes so that intact vesicles can be resolved. For example, following unoccluded application of vesicles and flexible vesicles incorporating sodium cholate (mean diameter 96 nm) to freshly excised human skin for 4-8 h, a large number of vesicles remained at the skin surface, particularly in the skin furrows, with very few intact vesicles in the stratum corneum. A smaller proportion of the flexible vesicles remained intact on the skin surface, and there was significantly more fluorescent probe in the deeper layers of the stratum corneum. However, the fluorescent signal was not associated with any vesicle-like shape, leading the authors to conclude that the flexible vesicle formulation components were contributing to the penetration enhancement. They also suggested that the choice of mouse skin with its thinner stratum corneum contributed to vesicle penetration reported in earlier studies.
Regardless of the mechanism, flexible nano-vesicles have been shown to enhance skin penetration of many compounds including photosensitizers for topical photodynamic therapy (PDT) [62], which requires effective delivery of compounds such as 5-aminolevulinic acid and temoporfin (mTHPC) that typically have very poor passive permeation, to cancer cells within the skin, followed by light activation to kill the cells. Dragicevic-Curic et al [52, 63] showed that the surface charge of flexible liposomes influenced the physicochemical properties, stability and permeation in human epidermal membranes in vitro, with skin deposition of mTHPC enhanced by all flexible vesicles in the following order: cationic > neutral > anionic > conventional liposomes. They suggested that cationic lipids most effectively interact with the negatively charged stratum corneum. Multiphoton tomography with fluorescence lifetime imaging microscopy (MPT-FLIM) showed that SECosomes (58 nm nanosomes composed of the cationic lipid 1,2-dioleoyl-3- trimethylammonium propane chloride (DOTAP), cholesterol, sodium cholate and 30% ethanol) delivered fluorescent siRNA into human epidermis after 1h, with NADH lifetime changes in the epidermal tissue suggesting siRNA cell penetration [54]. SECosome based delivery of siRNA to target human beta-defensin (hBD-2), which is highly up-regulated in psoriasis, in a bioengineered skin-humanized mouse model for psoriasis, lead to the recovery of transglutaminase activity, filaggrin expression and stratum corneum appearance that was similar to normal regenerated human skin [55]. Clearly flexible vesicles have demonstrated penetration enhancement for a range of compounds and provide useful formulations in skin delivery.
5.5. Application of Future Nanotechnologies
There is the potential to adopt a number of promising nanotechnologies to skin delivery. These include nanofiber-based dispersions generated by electrospinning [64, 65]. These amorphous solid dispersions (ASD) can not only improve dissolution behaviour of poorly water-soluble compounds but also provide programmable drug release profiles [66]. Electrospun fiber-based ASDs can maintain an incorporated active ingredient in the amorphous physical form for prolonged periods of time [64]. Whilst the primary focus of these ASDs is likely to be the generation of oral dosage forms for poorly water-soluble drugs, they also have potential for dosage forms delivered to the skin.
6. PHYSICAL ENHANCEMENT APPROACHES
6.1. Indirect Physical Methods
Indirect physical enhancement technologies (Fig. 4) involve the application of electrical (iontophoresis and electroporation), acoustic (sonophoresis), laser and magnetic energy have advanced through experimental evaluation to commercial development [67]. They apply an energy source to the skin surface to increase diffusivity of an applied solute in the stratum corneum.
Fig. (4).
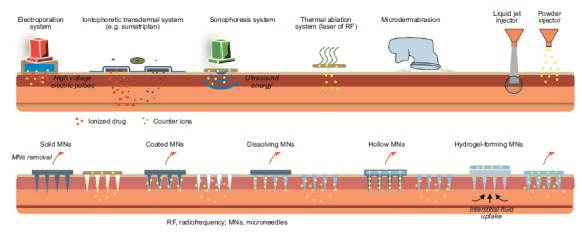
Physical penetration enhancement techniques.
6.1.1. Electroporation
Applies high intensity, high voltage (50-1500 V) electric pulses of short duration (10 μs to 10 ms) to form aqueous pores in the lipid bilayers of the stratum corneum, thus facilitating drug diffusion across the skin [68]. Enhanced skin penetration has been reported for drugs ranging from small e.g., fentanyl, doxorubicin [68-70], to high molecular weight such as LHRH, calcitonin, heparin, vaccines and FITC-dextrans with molecular weights up to 40 kDa [69, 71-76]. The main drawbacks of electroporation are the lack of quantitative delivery, cell death with high fields, pain and muscle contraction at the application site and potential damage to labile drugs, particularly peptides and proteins [77].
6.1.2. Iontophoresis
Applies a mild electric current (typically 0.1 to 1.0 mA/cm2) to increase skin permeation by three mechanisms: electromigration (ordered movement of ions within an applied electric field as described by Faraday’s law), electroosmosis (convective solvent flow in the anode-to-cathode direction due to the isoelectric point pI of 4 - 4.5 and negative charge of the skin) and enhanced passive diffusion (relatively minor role) [78-81]. Enhancement is greatest for suitably charged molecules. Iontophoretic molecular transport is determined by the intensity and duration of the applied current including the current density and continuous/pulsed waveform, and the area of electrode contact with the skin. Transport is also influenced by solute and vehicle factors including the solute concentration and molecular factors (charge/molecular mass ratio; volume, shape and charge distribution of the molecule), and the vehicle pH which influences both the solute and skin ionization (solute ionization affects mobility and electromigration; skin ionization determines the electroosmotic solvent flow) [82]. Iontophoresis is generally well tolerated, particularly when applied as a pulsed current profile that can allow time for the skin to depolarize and return to its resting state prior to initiation of the next pulse. Iontophoresis has been extensively investigated for enhancement of a wide range of molecules and in combination with other vehicle-based and physical enhancement methods [67, 78, 83]. A number of transdermal iontophoretic products have received regulatory approval including the LidoSite™ Topical System (lidocaine for local anaesthesia: Vyteris Inc., Fairlawn, NJ, USA), Ionsys™ (fentanyl iontophoretic transdermal system for patient-controlled analgesia; developed by Alza Corporation then acquired in sequence by Johnson & Johnson, Incline Therapeutics and The Medicines Company) and Zecuity™ (6.5 mg sumatriptan delivered over 4 h for migraine: NuPathe Inc./Teva). The Ionsys system was launched in Europe in 2008 but withdrawn due to corrosion. The Zecuity patch was introduced in September 2015 but sales were halted in June 2016 due to reports of burns at application site. There is clearly further development required to effectively exploit this technology.
6.1.3. Sonophoresis
Sonophoresis (phonophoresis) is the application of acoustic waves to facilitate transdermal delivery by cavitation (formation and oscillation of microbubbles), acoustic streaming, thermal effects and direct ultrasound pressure effects on the stratum corneum bilayers [84]. Polat et al. proposed that a microjet formed by the collapse of the cavitation bubbles in the application medium generates shock waves in the stratum corneum that perturb the barrier and increase permeability [85]. Krasovitsi et al. focused on modelling the applied ultrasonic pressure waves and suggested that their oscillation between compression and rarefaction transiently interrupts the continuity of the stratum corneum lipid bilayers to form pores through which applied drugs can diffuse [86]. Ultrasound-mediated skin delivery was initiated with high frequency ultrasound similar to that used in the treatment of musculoskeletal injuries [87-89] and progressed into low frequency ultrasound (20-100 kHz) which is reported to enhance permeation of a range of molecules including macromolecules such as insulin, interferon-γ and erythropoietin (5.8, 17 and 48 kDa respectively) across human skin [90]. The intensity and duration of ultrasound treatment is limited by the thermal effects on the skin therefore the combination of low and high-frequency ultrasound has been applied to generate a synergistic pressure effect without overheating the skin. Schoellhammer et al reported that a 4 min pre-treatment with dual high and low ultrasound frequencies (20 kHz and 1 MHz), in combination with the surfactant 1% sodium lauryl sulfate, enhanced the permeation of inulin and glucose across porcine skin by 3.81 and 13.6-fold respectively, compared to single frequency sonophoresis [91]. A bulky sonophoresis device named Sonoprep® was launched by Sontra Medical in 2004 and withdrawn 2007, then acquired by Echo Therapeutics who are also developing the Prelude® skin prep system but it has yet to be launched. Their application has been focused on preparing the skin for local anaesthetic drugs or glucose monitoring in diabetes management. Attempts have been made to develop wearable sonophoretic devices [92] but are yet to reach commercial applications.
6.1.4. Laser-assisted Delivery
Involves the generation of a photomechanical wave by laser ablation of a target medium consisting of a polymer such as black polystyrene placed on the surface of the skin. The medium undergoes rapid phase change (e.g. degradation or plasma formation) resulting in the production of a shockwave through the adjoining skin [93]. Unlike sonophoresis that oscillates between positive and negative pressure, there is no cavitation observed. The photomechanical wave is a unipolar compressive wave that disrupts the lipid arrangement within the stratum corneum intracellular space and also the cellular membranes. The technique has not progressed, with few publications in recent years.
6.1.5. Magnetophoresis
Is the enhancement of drug permeation by the application of a magnetic field, utilising diamagnetic repulsion of the molecule into the skin and possibly also causing transient stratum corneum barrier reduction [94, 95]. We have shown that pulsed electromagnetic fields (PEMF) increased the permeation of naltrexone hydrochloride across human epidermis by 6.5x compared to passive administration [95]. Gold nanoparticle (10 nm)-treated human skin exposed to a PEMF had 200x more gold nanoparticle positive pixels when visualised by multiphoton tomography with fluorescence lifetime imaging microscopy (MPM-FLIM), than skin exposed to gold nanoparticles without PEMF suggesting that the channels through which the nanoparticles move must be larger than 10 nm in diameter. An alternative magnetophoresis fabrication is a thin flexible polymer matrix containing multiple magnetic elements arranged to produce complex 3-dimensional magnetic gradients (40 mT peak magnetic field strength; 2 T/m2 total magnetic gradient: OBJ Ltd., Perth, Australia). In a recent pilot clinical trial, magnetophoresis enhanced transdermal delivery of key ground substance components (Lubricen™ gel containing chondroitin, glucosamine, hyaluronic acid) was shown to be equivalent to a commercially available transdermal non-steroidal anti-inflammatory formulation in controlling pain and facilitating movement in males with prior knee injury [96]. The first commercially available magnetophoresis-based enhancer consists of the magnetic array housed in an applicator that is used to enhance delivery of cosmetic actives in skin care products (SK-II and Olay brands, Procter & Gamble, Cincinnati, Ohio). Synergistic permeation enhancement of peptides has been demonstrated where it was hypothesised that the magnetic array increased flow in stratum corneum pores generated by microneedle pretreatment of human skin [97].
6.2. Direct Physical Methods
Direct physical enhancement methods can also be described as ‘minimally invasive’ methods and have in common an approach that involves creating a pore or hole in the stratum corneum barrier through which a drug molecule can be forced or flow to access the epidermis. All direct physical enhancement techniques increase skin permeation but their methods vary and include thermal, mechanical and pressure-based technologies (Fig. 4).
6.2.1. Microdermabrasion
Is utilised in dermatological and cosmeceutical practices for skin rejuvenation. It involves superficial disruption of the skin by instruments that generate air-propelled particles to ‘sandblast’ and simultaneously remove the skin by vacuum [98]. Microdermabrasion systems are designed for local disruption of the stratum corneum and superficial viable epidermis, resulting in minimal to no pain or bleeding and relatively fast recovery time [99, 100]. Although microdermabrasion is generally used for cosmetic skin rejuvenation, it has been applied with topical cosmetic products such as retinoic acid, vitamin C, and nicotinamide [101] and has also been investigated as a tool for enhancing delivery of therapeutic compounds into the skin including 5-fluorouracil, aminolevulinic acid, and clobetasol 17-propionate for local effects [102] and systemic drugs such as insulin [103]. Interestingly, whilst microdermabrasion increased the delivery of the hydrophilic drugs 5-fluorouracil and aminolevulinic acid (log partition coefficient -1.5 and -0.89 respectively), the flux of the lipophilic clobetasol 17-propionate (logP of 3.5) decreased following microdermabrasion [102]. This suggests that whilst removal of the lipid rich stratum corneum benefits penetration of hydrophilic compounds, it reduces penetration of lipophilic compounds. Drugs delivered post dermabrasion rely on passive diffusion within the viable skin tissue, potentially excluding lipophilic compounds that partition into the now removed stratum corneum lipid bilayers. Whilst removal of the stratum corneum by controlled microdermabrasion may be an effective means to enhance skin penetration and have utility in specific procedures such as PDT [104], it is unlikely to be a practical approach in most skin delivery applications.
6.2.2. Thermal Ablation
Applies extreme heat at the skin surface (≈300°C for microseconds) with no significant temperature rise in the underlying viable epidermis and deeper skin tissues [105], to vaporize portions of the stratum corneum to create micron-scale channels similar to those created by a microneedle array. This can be achieved by the application of arrays of microfabricated resistive [106] and radiofrequency [107, 108] electrical heating elements and by lasers. Examples are the ViaDor™ system (developed by TransPharma Medical, now owned by Syneron Medical Ltd., Israel) which creates 144 microchannels in 1cm2, and the Passport® system (developed by Altea Therapeutics, now owned by Nitto-Denko, Japan). In both cases, the device is used to ablate the skin prior to the application of a transdermal patch. Laser ablation is routinely applied in skin rejuvenation for dermatological (scarring, stretch marks) and cosmetic outcomes. In skin delivery investigations erbium:yttrium-gallium-garnet (Er:YAG) and yttrium scandium gallium garnet (YSGG) lasers, emitting at 2,790 nm and 2940 nm respectively, are most commonly used as they offer effective ablation of the stratum corneum with reduced thermal damage to the underlying viable tissue [109]. Whilst full coverage ablation provides greater penetration enhancement partial, coverage is more clinically relevant. Fractional laser ablation is applied to sub-millimetre regions to generate spots (typically 40 to 300 µm with densities between 50 and 600 cm-2) mimicking a microneedle array-type pattern [110-112]. The overall effect is to cause minimal thermal damage and promote fast healing times due to migration of neighbouring viable keratinocytes into the ablated cavities.
Penetration enhancement following fractional ablation with an Er:YAG laser of the relatively lipophilic imiquimod (logP 2.7) and hydrophilic aminolevulinic acid (logP -1.5) was 8.83-fold and 41.23-fold respectively [113], again demonstrating that removal of the stratum corneum is more effective for hydrophilic than lipophilic compounds. Fractional laser ablation has also been shown to allow effective delivery of large molecules such as 10 kDa dextran, siRNA, plasmid DNA and vaccines [110, 114]. There will need to be significant technological developments as current bulky and expensive laser devices are a significant limitation for their further development in the transdermal delivery field.
6.2.3. Biolistic Injectors
Deliver a liquid or particulate payload to the skin surface at high velocity to breach the stratum corneum. Liquid jet injectors apply high pressure to eject through a small nozzle, at velocity 100-200 m/s, with the delivered volume and penetration depth dependent on the velocity and microjet size [115]. Deeper penetration is achieved with higher velocities, but there is also greater potential for splash back of solution from the skin surface and damage to the skin tissues [116]. Volumes in the nanolitre range minimise damage and splashback [117]. The skin is pre-ablated to facilitate penetration of the propelled liquid. Jang and colleagues used a 250 µs ER:YAG laser pulse laser to generate vapour bubbles and downstream shockwaves that propelled a microjet of liquid containing epidermal growth factor and human growth hormone into pre-ablated pig skin [118, 119]. Microjet velocities of 23.0 to 50.6 m/s provided effective delivery of epidermal growth factor and human growth hormone in porcine skin, as determined by gene expression of keratinocyte laminin and fibroblast elastin, respectively. A subsequent refinement of the procedure has involved a pre-ablation step prior to microjet generation, both achieved by the same laser [120]. The advantage is that pre-ablation is targeted to finite areas, with the microjet then bombarding those defined areas. Effective drug delivery efficiency is achieved with much reduced tissue damage and more rapid reepithelialisation.
The original biolistic particle delivery system, or gene gun, was designed for delivering exogenous DNA (transgenes), typically as a particle of a heavy metal coated with plasmid DNA, into plant cells. This has been developed into biolistic injectors delivering a ‘shot gun’ burst of nano- or micro-particles into the skin, that have been effective for the immunisation of antigens including influenza, malaria and also anti-cancer applications [121-124]. Solid or dense spheroidal particles in the sub-micron size range are typically used for biolistic particle delivery [125], although porous particles can provide options for increased drug loading and controlled release [121]. For both liquid and particulate biolistic injection technologies, their most likely adoption is an alternative to conventional injection such as vaccination. Examples of their clinical role in this field are described later.
6.2.4. Microneedle
Arrays are the direct penetration enhancement approach that has garnered most attention as an alternative to conventional hypodermic and subcutaneous injections. They have been the subject of extensive investigation in academia and industry and their development towards the clinic is well advanced, particularly for vaccination [126, 127]. Microneedle array fabrications vary from the so called ‘poke and patch’ to the inclusion of their payload on the surface or within the projections, but they have in common the mechanism of poking an array of small, sharp projections into the skin to breech the stratum corneum barrier. Microneedle projections typically range from 25 to 2000 μm in height, 50 to 250 μm in base width and 1 to 25 μm in tip diameter [29], so that they do not contact the nerve endings or cutaneous blood vessels thereby providing pain-free administration [128] and avoiding the potential of transmitting blood-borne infection [129]. The shape, size and material of the microneedles must be such that the projections will overcome the elasticity of the skin to enter the epidermis to sufficient depth and will remain intact to facilitate removal, unless they are designed to dissolve within the skin. Pores caused by microneedle projections of a range of geometries have been shown to close within 2h unless occluded, which can increase closure time to over 24h [130]. There are five main types (Fig. 4): solid, coated, dissolvable, hollow and hydrogel swellable microneedles, the latter designed to remain in situ and facilitate diagnosis.
Solid microneedles employ the ‘poke and patch’ approach whereby the microneedle array is used as a pre-treatment to create microchannels in the epidermis, then removed and a drug-laden patch or other suitable formulation is applied to release drug that can diffuse into the viable epidermis via the transient channels. Solid microneedles have been fabricated from a number of materials including metals, silicon and polymers [126].
Coated microneedles provide the advantage of a one-step application but dosage is limited to the quantity that can be coated onto the total surface area of the microneedle projections [131]. Coated microneedles have attracted particular interest for macromolecules such as vaccines [132-136].
Dissolvable microneedles approach, are composed of materials that form sharp projections with the payload dissolved or suspended within, allowing them to be poked into and then dissolve within the skin to release their payload. At completion there is no need to remove and dispose of the microneedle array. Dissolving microneedles composed of varied materials have been very widely investigated for a range of applications from caffeine delivery to combat obesity [137] and 5-aminolevulinic acid for PDT [138] to macromolecules such as insulin [139, 140] and vaccines [141-143], and diagnostic monitoring [144].
Hollow microneedles are akin to an array of ting hypodermic needles that allow ‘poke and flow’ of drug solution into the skin [145]. They require more complex microneedle fabrication and pressurized assistance to generate and regulate flow [129].
Swellable microneedles consist of suitable hydrogels that can be poked into the skin and will then swell and remain in situ. Water or skin interstitial fluid diffuses into the microneedle array leading to controlled swelling that permits the diffusion of drug molecules [146-148] applied in an adhesive patch into the skin, or the egress of physiological chemicals such as glucose from the skin to permit diagnostic monitoring [149, 150].
The range of materials, fabrications, manufacturing and engineering principles in the design and development of microneedles is beyond the scope of this article, but excellent reviews are available elsewhere [142, 151, 152].
Human studies have demonstrated that consistent microneedle application/penetration to the skin site of application is essential for reliable drug delivery [153]. This can be achieved either manually by simply applying thumb pressure or by using an applicator (examples are the Zosano Pharma, Vaxxas and Corium International microneedle technologies). Studies have shown that microneedle arrays are well received by consumers and can be administered equally well by a range of consumers and health professionals [154, 155]. At present there are no true microneedle devices on the market. The closest technologies are the Micronjet® 600 (NanoPass Technologies Ltd., Israel) and Soluvia® (Becton Dickinson, USA) that have both demonstrated better immune response for influenza vaccine compared to IM injection. In both cases these are essentially micronized injection devices that allow intradermal administration from pre-fillable microinjection systems consisting of small needles attached to a conventional syringe [156]. Although there are significant manufacturing and regulatory hurdles that will need to be overcome, there is clear future potential for microneedle based skin delivery in the management of chronic conditions such as diabetes [157] and in global vaccination programs.
6.2.5. High Aspect Ratio Elongated Microparticles
Elongated microparticles (EMP) for enhanced drug delivery developed by Prow’s research group in Australia [158, 159], are made of cylindrical shaped solid silica with an average width of 28 ± 11 µm and average length between 120.1 and 483.2 µm. EMP can be applied as a pretreatment (akin to the ‘poke and patch’) or mixed with existing formulations and massaged onto the skin to facilitate permeation of the simultaneously applied active in the formulation. The EMP/skin surface contact angle is optimized by applying the EMP/formula- tion with a 3D printed micro-textured applicator that ensures low angular penetration within the epidermis, and penetrate to the dermal-epidermal junction. EMP enhancement ratios of 8.5- and 8.8-fold for Vitamin E (3H-a-tocopherol acetate) and Vitamin B3 (3H-nicotinamide) respectively compared to passive administration in excised human skin were reported [158]. They have recently demonstrated the successful combination of a nanoemulsion and EMP to deliver lipophilic compounds into the skin [160]. Normal transepidermal turnover and desquamation pushes the microparticles out of healthy skin within 3 weeks. An advantage over microneedle and other array-type enhancement technologies is that the EMP can be applied to varied sizes and shapes of application site.
6.3. Combinations of Enhancement Approaches
As with all techniques that act by creating pores in the stratum corneum, an important consideration is how the properties of the drug and vehicle applied within those pores influence passive diffusion within the skin. Many different combinations of penetration enhancement approaches have been explored with the aim of developing low cost, efficient and effective technologies capable of increased and/or targeted delivery to and via the skin. Examples are: the combination of indirect physical enhancement energy to improve diffusion within pores or into the tissues in contact with pores generated by direct physical enhancers (microneedles with magnetophoresis [161] and iontophoresis [162]); and nanosystems to increase drug solubility or control release within the skin after access generated by physical enhancer [163].
6.4. Skin Delivery: Technologies Creating the Future?
The application of effective penetration enhancement technologies can offer new opportunities for delivery of a much wider range of therapeutic compounds to and across the skin. Transiently overcoming the skin barrier also offers opportunities for diagnostic technologies applied to the skin. We have focused on a couple of innovative directions utilising the skin: vaccination and smart technologies that combine diagnosis and responsive therapy.
6.5. Skin Delivery for Vaccination
The opportunity to deliver vaccines via the skin is an alternative to conventional invasive and painful intramuscular or subcutaneous injections with hypodermic needle and syringe associated with the risk of blood-borne disease transmission needlestick injuries (i.e. accidental punctures by contaminated needles) [164]. The skin layers are rich in immune cells such as the epidermal Langerhans cells and the dermal dendritic cells with antigen-presenting capacity [165] with the opportunity to generate a stronger immune response compared to deeper injection [166], and consequently reduce the required dose and associated cost. For example, in our work comparing immunization of mice with the influenza vaccine Fluvax by microprojection array to the buccal mucosa and skin, verses oral and IM delivery, resultant IgA levels and immune response were significantly higher for skin delivery (Fig. 5: [167]). To deliver vaccines, which are typically macromolecules, research and commercialization has focused on the various non-invasive penetration enhancement approaches described above. For example the PharmaJet needle-free jet injector uses a spring-powered, hand-held injector to shoot a precise fluid stream containing the vaccine through the skin and into the muscle [168, 169], with pressure that is reported to feel like the snap of a rubber band [170]. Afluria® influenza vaccine was approved by the US FDA in 2014 and initial uptake has been positive. PharmaJet needle-free injector intradermal delivery has also been developed for poliovirus [171] and MMR (measles, mumps, rubella), and is in use in a number of countries (https://pharmajet.com).
Fig. (5).
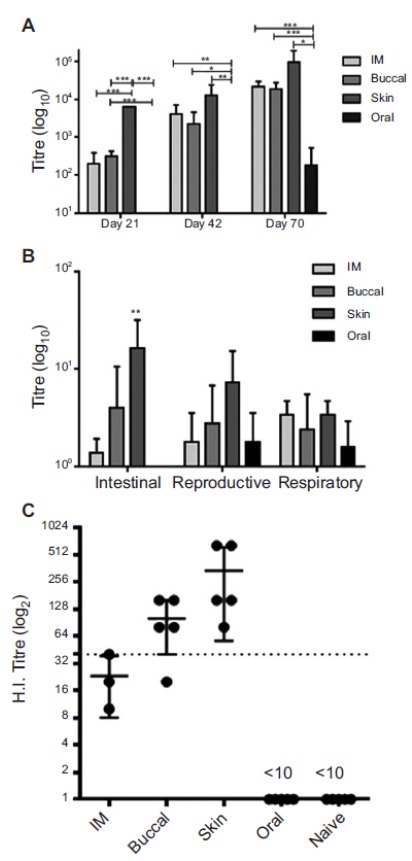
Anti-influenza immunogenicity of Fluvax® 2010 coated Nanopatches applied to the buccal mucosa. (A) Total serum IgG responses at various times after immunisation and boost. (B) IgA responses in samples extracted from faeces, female reproductive tract and nasal passages after the final immunisation (Day 70). (C) Haemagglutination inhibition titres against the A/Perth/16/2009 like influenza strain in serum collected 21 days after the initial immunisation. The black line at a HI titre of 40 represents the accepted minimum that correlates with protection in humans. In all panels bars represent the mean and standard deviation from the mean are shown. Significance of p ≤ 0.01 (***), p = 0.01-0.05 (**) or p = 0.05-0.1 (*) is indicated. In all immune studies n = 5 animals were used per group. Reproduced with permission from [167].
Alternative minimally invasive vaccination methods have been developed with most focus being on the use of microneedles for the delivery of influenza antigens and DNA vaccines [151, 172-174]. A microneedle patch for the delivery of inactivated influenza vaccine has reached the clinical trial stage (Phase I) in humans (NCT02438423), led by Mark Prausnitz’s research team (Georgia Institute of Technology, GA, USA). Administration of a 1 cm2 array of 100 dissolvable microneedles (composed of sucrose and polyvinyl alcohol) that detached and dissolved within about 20 mins, was well tolerated and provided titres and seroconversion rates similar to intramuscular injections [168]. The same group has received funding from the Bill and Melinda Gates Foundation for the development of a polio microneedle patch and Phase I clinical trials in humans. The NanopatchTM (Vaxxas, Australia) is an extension of the microneedle approach where the array contains 10,000-20,000 projections per cm2 (~110-250 µm in length, ~20-40 µm in base width) with the vaccine dry coated onto their surface. It has shown promise during animal clinical trials for vaccination against influenza [175, 176], herpes simplex virus [177], human papillomavirus-associated cervical cancer [178] and poliomyelitis [179, 180]. Self-administration of microneedle patches could improve vaccination coverage [181] and reduce administration costs, and avoiding the need for cold storage has major implications for vaccination in developing countries.
Lademann’s group [181] explored the potential to deliver vaccines by topical application of microparticles that penetrate into the hair follicles. The infundibulum within the hair follicle (400-600 μm deep) is lined with a particularly dense network of dendridic cells [182] making it the most immunologically active region, therefore this was specifically targeted for delivery. As they had previously demonstrated that particles of different sizes penetrate follicles to different depths, the vaccine carriers were of 1 mm diameter to target the infundibulum. They showed that their 1 mm silicon dioxide microparticles efficiently penetrated the hair follicles to deliver vaccine constituents to dendridic cells in the infundibulum within 1 hour of topical application. They also showed that particles remained as a depot within the follicle for up to 10 days contrasting with particles on the stratum corneum that were rapidly removed after about 1 day. Follicular delivery of vaccine carried on microparticles provides another potential approach to target the body’s largest immune organ.
6.6. Smart Wearable Technologies for Diagnosis and Treatment
The future of skin delivery lies with the technological innovations being developed for enhanced skin penetration and site-specific targeting to extend the range of solutes that can be successfully delivered and thereby the clinical applications of skin delivery. Technological innovations in skin delivery include advanced transdermal patch fabrications, polymeric-based nanoparticles, lipid-based flexible nano-vesicles, energy-based enhancers and minimally invasive delivery systems. An in-depth discussion of these technologies is not the focus of this manuscript, but we have highlighted some examples where skin delivery may provide significant advances in healthcare in the future.
There have been considerable advances in the use of microneedle patches to monitor and manage diabetes, one of the major global health challenges. The holy grail of diabetes management is a non-invasive feedback system combining glucose monitoring and responsive drug delivery. Two recent studies have reported the non-invasive management of diabetes using microneedle patches for the monitoring of glucose blood levels and subsequent release of the therapeutic active. In the first study, a 6 mm2 array of 121 conical microneedles contained polymer nanoparticles loaded with insulin and glucose oxidase enzyme which access the interstitial fluid when inserted into the skin (Fig. 6A) [183]. A rise in glucose blood level (hyperglycaemia) causes an increase in enzymatic activity, converting glucose to gluconic acid and consuming oxygen in the process, thereby creating a local hypoxic micro-environment within the microneedle projections. This hypoxic environment triggers dissociation of the polymer nanoparticles with subsequent release of their insulin. The ‘smart insulin patch’ effectively regulated blood glucose levels in in vivo trials with mouse models of chemically induced type 1 diabetes [183]. The second approach utilizes a patch that absorbs sweat from the skin, contains sensors for glucose, pH, humidity and temperature, combined with polymeric microneedles containing the antidiabetic drug metformin (Fig. 6B) [184, 185]. The microneedles are coated with the hydrophobic compound tridecanoic acid to protect them from skin moisture and prevent drug release. Blood glucose is monitored once sufficient sweat is generated, typically after 20-30 min. When elevated glucose level is detected, it triggers the heater embedded in the patch, warming the microneedles above the transition temperature of tridecanoic acid (~41°C), exposing the microneedle polymer to interstitial water and releasing the metformin. The connection of the patch to a portable electrochemical analyser also enabled wireless control while at the same time transmitting collected data for further treatment optimization [184, 185]. In addition to a robust set of in vivo data obtained from an in vivo genetically diabetic mouse model, the developed transdermal drug delivery system was shown to be flexible, robust and conforms to the skin. This group have further developed their technology into a wearable/ disposable sweat-based glucose monitoring device integrated with a feedback transdermal drug delivery module, with more elegant fabrication providing improvements in wearability and accuracy. This approach has also been applied for monitoring tremor frequencies (seen in movement disorders such as epilepsy and Parkinson’s disease) through the recording of muscle activity [186]. The collected data could then be use to activate a microneedle delivered feedback therapy (e.g. anti-Parkinson’s disease drug) through thermal stimuli [186]. It is likely that we will see this approach of wearable sensor/delivery patch technology applied to a range of chronic conditions, particularly where potent drug therapies are available, with devices in human trials in the future.
Fig. (6).
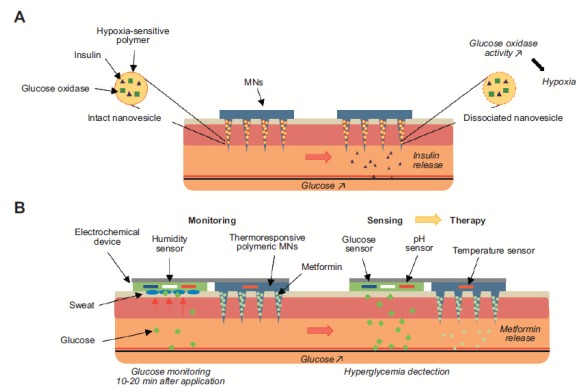
a) The ‘smart insulin patch’ effectively regulates blood glucose levels by sensing hypoxia in the local microenvironment. b) A sophisticated system to sense glucose, pH, humidity and temperature by absorbing sweat and release antidiabetic drug upon trigger.
CONCLUSION
The skin is a key site for local and systemic drug delivery. It has the unique qualities of being easily accessible yet relatively impermeable. This allows products from a wide range of formulations to be applied to the skin and then removed as required. Products for skin application have evolved from simple solutions and ointments to multiphase, nanotechnologies and assisted technologies. It is essential that the increasingly sophisticated products being developed address market, design, safety and sensorial requirements for the range of situations in which they are applied to the skin.
ACKNOWLEDGEMENTS
The authors thank the National Health & Medical Research Council of Australia (NHMRC), Australian Research Council (ARC) and the US FDA for their support of our work on topical absorption over many years.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
Michael Roberts would like to specifically acknowledge the support by grants APP1107356 and APP1055176 from the NHMRC.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Geller M.J. Ancient Babylonian medicine. Malden: Wiley-Blackwell; 2010. [Google Scholar]
- 2.Selwyn S. The topical treatment of skin infection. In: Maibach H.I., Aly R., editors. Skin Microbiology. Relevance to Clinical Infection. New York: Springer-Verlag; 1981. pp. 317–328. [Google Scholar]
- 3.Ebbell B. The Papyrus Ebers: the greatest Egyptian medical document. Copenhagen: Levin & Munksgaard; 1937. [Google Scholar]
- 4.Moghimi H.R., Shafizade A., Kamlinejad M. Drug delivery systems in Iranian traditional pharmacy (in Persian). Traditional Medicine and Materia Medica Research Center, SBMU, Tehran, Iran: 2011. [Google Scholar]
- 5.Morgan W.F. Poisoning by the external application of belladonna. BMJ. 1866;2:621. [Google Scholar]
- 6.Laws G.E. Nitroglycerin head. J. Am. Med. Assoc. 1910;54:793. [Google Scholar]
- 7.Schwenkenbecker A. Das Absorptionsvermögen der Haut. Archiv. für Physiol. 1904;1(2):121–165. [Google Scholar]
- 8.Rein H. Experimental electroendosmotic studies on living human skin. Z. Biol. 1924;81:124. [Google Scholar]
- 9.Moore C.R., Lamar J.K., Beck N. Cutaneous absorption of sex hormones. J. Am. Med. Assoc. 1938;111:11–14. [Google Scholar]
- 10.Zondek B. Cutaneous application of follicular hormone. Lancet. 1938;1:1474. [Google Scholar]
- 11.Zondek B. The excretion of halogenated phenols and their use in the treatment of urogenital infections. J. Urol. 1942;48:747–758. [Google Scholar]
- 12.Fox M.J., Leslie C.L. Treatment of Raynaud’s diseases with nitroglycerine. Wis. Med. J. 1948;47:855–858. [PubMed] [Google Scholar]
- 13.Lund F. Percutaneous nitroglycerin treatment in cases of peripheral circulatory disorders, especially Raynaud’s disease. Acta Med. Scand. 1948;131:196–206. doi: 10.1111/j.0954-6820.1948.tb12036.x. [DOI] [PubMed] [Google Scholar]
- 14.Rothman S. The principles of percutaneous absorption. J. Lab. Clin. Med. 1943;28:1305–1321. [Google Scholar]
- 15.Higuchi T. Physical chemical analysis of percutaneous absorption processes. J. Soc. Cosmet. Chem. 1960;11:85–97. [Google Scholar]
- 16.Roberts M.S., Anderson R.A., Swarbrick J. Permeability of human epidermis to phenolic compounds. J. Pharm. Pharmacol. 1977;29:677–683. doi: 10.1111/j.2042-7158.1977.tb11434.x. [DOI] [PubMed] [Google Scholar]
- 17.Katz M., Poulsen B.J. Absorption of drugs through the skin. In: Brodie B.B., Gilette J., editors. Springer Verlag. Berlin: 1971. pp. 103–174. (Handbook of Experimental Pharmacology). [Google Scholar]
- 18.Scheuplein R.J., Blank I.H. Permeability of the skin. Physiol. Rev. 1971;51:702–747. doi: 10.1152/physrev.1971.51.4.702. [DOI] [PubMed] [Google Scholar]
- 19.Roberts M.S., Anderson R.A., Swarbrick J., Moore D.E. The percutaneous absorption of phenolic compounds: the mechanism of diffusion across the stratum corneum. J. Pharm. Pharmacol. 1978;30:486–490. doi: 10.1111/j.2042-7158.1978.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 20.Michaels A.S., Chandrasekaran S.K., Shaw J.E. Drug permeation through human skin: Theory and in vitro experimental measurement. AIChE J. 1975;21:985–996. [Google Scholar]
- 21.Pastore M.N., Kalia Y.N., Horstmann M., Roberts M.S. Transdermal patches: History, development and pharmacology. Br. J. Pharmacol. 2015;172:2179–2209. doi: 10.1111/bph.13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isaac M., Holvey C. Transdermal patches: The emerging mode of drug delivery system in psychiatry. Ther. Adv. Psychopharmacol. 2012;2:255–263. doi: 10.1177/2045125312458311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McAfee D.A., Hadgraft J., Lane M.E. Rotigotine: The first new chemical entity for transdermal drug delivery. Eur. J. Pharm. Biopharm. 2014;88:586–593. doi: 10.1016/j.ejpb.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Crank J. The mathematics of diffusion. 2nd ed. Oxford, UK: Clarendon Press; 1979. [Google Scholar]
- 25.Yousef S., Liu X., Mostafa A., Mohammed Y., Grice J.E., Anissimov Y.G., Sakran W., Roberts M.S. estimating maximal in vitro skin permeation flux from studies using non-sink receptor phase conditions. Pharm. Res. 2016;33:2180–2194. doi: 10.1007/s11095-016-1955-8. [DOI] [PubMed] [Google Scholar]
- 26.Roberts M.S., Mohammed Y., Pastore M.N., Namjoshi S., Yousef S., Alinaghi A., Haridass I.N., Abd E., Leite-Silva V.R., Benson H., Grice J.E. Topical and cutaneous delivery using nanosystems. J. Control. Release. 2017;247:86–105. doi: 10.1016/j.jconrel.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 27.Nastiti C.M.R.R., Ponto T., Abd E., Grice J.E., Benson H.A.E., Roberts M.S. Topical nano and microemulsions for skin delivery. Pharmaceutics. 2017;9:E37. doi: 10.3390/pharmaceutics9040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ita K.B. Chemical penetration enhancers for transdermal drug delivery-success and challenges. Curr. Drug Deliv. 2015;12:645–651. doi: 10.2174/1567201812666150804104600. [DOI] [PubMed] [Google Scholar]
- 29.Alkilani A.Z., McCrudden M.T., Donnelly R.F. Transdermal drug delivery: Innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics. 2015;7:438–470. doi: 10.3390/pharmaceutics7040438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McClements D.J. Nanoemulsions versus microemulsions: Clarification of critical differences. Soft Matter. 2012;8:1719–1729. [Google Scholar]
- 31.Abd E., Namjoshi S., Mohammed Y.H., Roberts M.S., Grice J.E. Synergistic skin penetration enhancer and nanoemulsion formulations promote the human epidermal permeation of caffeine and naproxen. J. Pharm. Sci. 2016;105:212–220. doi: 10.1002/jps.24699. [DOI] [PubMed] [Google Scholar]
- 32.Muller R.H., Radtke M., Wissing S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002;54(Suppl. 1):S131–S155. doi: 10.1016/s0169-409x(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 33.Jenning V., Gysler A., Schafer-Korting M., Gohla S.H. Vitamin A loaded solid lipid nanoparticles for topical use: Occlusive properties and drug targeting to the upper skin. Eur. J. Pharm. Biopharm. 2000;49:211–218. doi: 10.1016/s0939-6411(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y.T., Wu Z.H., Zhang K., Zhao J.H., Ye B.N., Feng N.P. An in vitro and in vivo comparison of solid and liquid-oil cores in transdermal aconitine nanocarriers. J. Pharm. Sci. 2014;103:3602–3610. doi: 10.1002/jps.24152. [DOI] [PubMed] [Google Scholar]
- 35.Wissing S.A., Muller R.H. The influence of solid lipid nanoparticles on skin hydration and viscoelasticity--in vivo study. Eur. J. Pharm. Biopharm. 2003;56:67–72. doi: 10.1016/s0939-6411(03)00040-7. [DOI] [PubMed] [Google Scholar]
- 36.Khurana S., Bedi P.M., Jain N.K. Preparation and evaluation of solid lipid nanoparticles based nanogel for dermal delivery of meloxicam. Chem. Phys. Lipids. 2013;175-176:65–72. doi: 10.1016/j.chemphyslip.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Shrotriya S., Ranpise N., Satpute P., Vidhate B. Skin targeting of curcumin solid lipid nanoparticles-engrossed topical gel for the treatment of pigmentation and irritant contact dermatitis. Artif. Cells Nanomed. Biotechnol. 2018;46:1471–1482. doi: 10.1080/21691401.2017.1373659. [DOI] [PubMed] [Google Scholar]
- 38.Jin S.E., Kim C.K. Charge-mediated topical delivery of plasmid DNA with cationic lipid nanoparticles to the skin. Colloids Surf. B Biointerfaces. 2014;116:582–590. doi: 10.1016/j.colsurfb.2014.01.053. [DOI] [PubMed] [Google Scholar]
- 39.Xia Q., Saupe A., Muller R.H., Souto E.B. Nanostructured lipid carriers as novel carrier for sunscreen formulations. Int. J. Cosmet. Sci. 2007;29:473–482. doi: 10.1111/j.1468-2494.2007.00410.x. [DOI] [PubMed] [Google Scholar]
- 40.Pople P.V., Singh K.K. Development and evaluation of colloidal modified nanolipid carrier: Application to topical delivery of tacrolimus. Eur. J. Pharm. Biopharm. 2011;79:82–94. doi: 10.1016/j.ejpb.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 41.Pople P.V., Singh K.K. Development and evaluation of colloidal modified nanolipid carrier: application to topical delivery of tacrolimus, Part II--in vivo assessment, drug targeting, efficacy, and safety in treatment for atopic dermatitis. Eur. J. Pharm. Biopharm. 2013;84:72–83. doi: 10.1016/j.ejpb.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 42.Patlolla R.R., Desai P.R., Belay K., Singh M.S. Translocation of cell penetrating peptide engrafted nanoparticles across skin layers. Biomaterials. 2010;31:5598–5607. doi: 10.1016/j.biomaterials.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shah P.P., Desai P.R., Channer D., Singh M. Enhanced skin permeation using polyarginine modified nanostructured lipid carriers. J. Control. Release. 2012;161:735–745. doi: 10.1016/j.jconrel.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cevc G. Transfersomes, liposomes and other lipid suspensions on the skin: Permeation enhancement, vesicle penetration, and transdermal drug delivery. Crit. Rev. Ther. Drug Carrier Syst. 1996;13:257–388. doi: 10.1615/critrevtherdrugcarriersyst.v13.i3-4.30. [DOI] [PubMed] [Google Scholar]
- 45.De Marco Almeida F., Silva C.N., de Araujo Lopes S.C., Santos D.M., Torres F.S., Cardoso F.L., Martinelli P.M., da Silva E.R., de Lima M.E., Miranda L.A.F., Oliveira M.C. Physicochemical characterization and skin permeation of cationic transfersomes containing the synthetic peptide PnPP-19. Curr. Drug Deliv. 2018;15:1064–1071. doi: 10.2174/1567201815666180108170206. [DOI] [PubMed] [Google Scholar]
- 46.Manconi M., Manca M.L., Caddeo C., Valenti D., Cencetti C., Diez-Sales O., Nacher A., Mir-Palomo S., Terencio M.C., Demurtas D., Gomez-Fernandez J.C., Aranda F.J., Fadda A.M., Matricardi P. Nanodesign of new self-assembling core-shell gellan-transfersomes loading baicalin and in vivo evaluation of repair response in skin. Nanomedicine (Lond.) 2018;14:569–579. doi: 10.1016/j.nano.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Garg V., Singh H., Bimbrawh S., Singh S.K., Gulati M., Vaidya Y., Kaur P. Ethosomes and transfersomes: Principles, perspectives and practices. Curr. Drug Deliv. 2017;14:613–633. doi: 10.2174/1567201813666160520114436. [DOI] [PubMed] [Google Scholar]
- 48.Godin B., Touitou E. Ethosomes: New prospects in transdermal delivery. Crit. Rev. Ther. Drug Carrier Syst. 2003;20:63–102. doi: 10.1615/critrevtherdrugcarriersyst.v20.i1.20. [DOI] [PubMed] [Google Scholar]
- 49.Das S.K., Chakraborty S., Roy C., Rajabalaya R., Mohaimin A.W., Khanam J., Nanda A., David S.R. Ethosomes as novel vesicular carrier: An overview of the principle, preparation and its applications. Curr. Drug Deliv. 2018;15:795–817. doi: 10.2174/1567201815666180116091604. [DOI] [PubMed] [Google Scholar]
- 50.Muzzalupo R., Tavano L., Cassano R., Trombino S., Ferrarelli T., Picci N. A new approach for the evaluation of niosomes as effective transdermal drug delivery systems. Eur. J. Pharm. Biopharm. 2011;79:28–35. doi: 10.1016/j.ejpb.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 51.Manosroi J., Khositsuntiwong N., Manosroi W., Gotz F., Werner R.G., Manosroi A. Potent enhancement of transdermal absorption and stability of human tyrosinase plasmid (pAH7/Tyr) by Tat peptide and an entrapment in elastic cationic niosomes. Drug Deliv. 2013;20:10–18. doi: 10.3109/10717544.2012.742937. [DOI] [PubMed] [Google Scholar]
- 52.Dragicevic-Curic N., Scheglmann D., Albrecht V., Fahr A. Temoporfin-loaded invasomes: Development, characterization and in vitro skin penetration studies. J. Control. Release. 2008;127:59–69. doi: 10.1016/j.jconrel.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 53.Shah S.M., Ashtikar M., Jain A.S., Makhija D.T., Nikam Y., Gude R.P., Steiniger F., Jagtap A.A., Nagarsenker M.S., Fahr A. LeciPlex, invasomes, and liposomes: A skin penetration study. Int. J. Pharm. 2015;490:391–403. doi: 10.1016/j.ijpharm.2015.05.042. [DOI] [PubMed] [Google Scholar]
- 54.Geusens B., Van Gele M., Braat S., De Smedt S.C., Stuart M.C., Prow T.W., Sanchez W., Roberts M.S., Sanders N., Lambert J. Flexible nanosomes (SECosomes) enable efficient siRNA delivery in cultured primary skin cells and in the viable epidermis of ex vivo human skin. Adv. Funct. Mater. 2010;20:4077–4090. [Google Scholar]
- 55.Bracke S., Carretero M., Guerrero-Aspizua S., Desmet E., Illera N., Navarro M., Lambert J., Del Rio M. Targeted silencing of DEFB4 in a bioengineered skin-humanized mouse model for psoriasis: Development of siRNA SECosome-based novel therapies. Exp. Dermatol. 2014;23:199–201. doi: 10.1111/exd.12321. [DOI] [PubMed] [Google Scholar]
- 56.Mura S., Manconi M., Fadda A.M., Sala M.C., Perricci J., Pini E., Sinico C. Penetration enhancer-containing vesicles (PEVs) as carriers for cutaneous delivery of minoxidil: In vitro evaluation of drug permeation by infrared spectroscopy. Pharm. Dev. Technol. 2013;18:1339–1345. doi: 10.3109/10837450.2012.685661. [DOI] [PubMed] [Google Scholar]
- 57.Manconi M., Caddeo C., Sinico C., Valenti D., Mostallino M.C., Biggio G., Fadda A.M. Ex vivo skin delivery of diclofenac by transcutol containing liposomes and suggested mechanism of vesicle-skin interaction. Eur. J. Pharm. Biopharm. 2011;78:27–35. doi: 10.1016/j.ejpb.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 58.Manca M.L., Manconi M., Zaru M., Valenti D., Peris J.E., Matricardi P., Maccioni A.M., Fadda A.M. Glycerosomes: Investigation of role of 1,2-dimyristoyl-sn-glycero-3-phosphatidycholine (DMPC) on the assembling and skin delivery performances. Int. J. Pharm. 2017;532:401–407. doi: 10.1016/j.ijpharm.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 59.Dreier J., Sorensen J.A., Brewer J.R. Superresolution and fluorescence dynamics evidence reveal that intact liposomes do not cross the human skin barrier. PLoS One. 2016;11:e0146514. doi: 10.1371/journal.pone.0146514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brewer J., Bloksgaard M., Kubiak J., Sorensen J.A., Bagatolli L.A. Spatially resolved two-color diffusion measurements in human skin applied to transdermal liposome penetration. J. Invest. Dermatol. 2013;133:1260–1268. doi: 10.1038/jid.2012.461. [DOI] [PubMed] [Google Scholar]
- 61.Cevc G., Gebauer D. Hydration-driven transport of deformable lipid vesicles through fine pores and the skin barrier. Biophys. J. 2003;84:1010–1024. doi: 10.1016/S0006-3495(03)74917-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dragicevic-Curic N., Fahr A. Liposomes in topical photodynamic therapy. Expert Opin. Drug Deliv. 2012;9:1015–1032. doi: 10.1517/17425247.2012.697894. [DOI] [PubMed] [Google Scholar]
- 63.Dragicevic-Curic N., Grafe S., Gitter B., Winter S., Fahr A. Surface charged temoporfin-loaded flexible vesicles: In vitro skin penetration studies and stability. Int. J. Pharm. 2010;384:100–108. doi: 10.1016/j.ijpharm.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 64.Yu D.G., Li J.J., Williams G.R., Zhao M. Electrospun amorphous solid dispersions of poorly water-soluble drugs: A review. J. Control. Release. 2018;292:91–110. doi: 10.1016/j.jconrel.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 65.Li J.J., Yang Y.Y., Yu D.G., Du Q., Yang X.L. Fast dissolving drug delivery membrane based on the ultra-thin shell of electrospun core-shell nanofibers. Eur. J. Pharm. Sci. 2018;122:195–204. doi: 10.1016/j.ejps.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 66.Hai T., Wan X., Yu D-G., Wang K., Yang Y., Liu Z-P. Electrospun lipid-coated medicated nanocomposites for an improved drug sustained-release profile. Mater. Des. 2019;162:70–79. [Google Scholar]
- 67.Mitragotri S. Devices for overcoming biological barriers: The use of physical forces to disrupt the barriers. Adv. Drug Deliv. Rev. 2013;65:100–103. doi: 10.1016/j.addr.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 68.Denet A.R., Vanbever R., Preat V. Skin electroporation for transdermal and topical delivery. Adv. Drug Deliv. Rev. 2004;56:659–674. doi: 10.1016/j.addr.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 69.Blagus T., Markelc B., Cemazar M., Kosjek T., Preat V., Miklavcic D., Sersa G. In vivo real-time monitoring system of electroporation mediated control of transdermal and topical drug delivery. J. Control. Release. 2013;172:862–871. doi: 10.1016/j.jconrel.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 70.Prausnitz M.R. A practical assessment of transdermal drug delivery by skin electroporation. Adv. Drug Deliv. Rev. 1999;35:61–76. doi: 10.1016/s0169-409x(98)00063-5. [DOI] [PubMed] [Google Scholar]
- 71.Bommannan D.B., Tamada J., Leung L., Potts R.O. Effect of electroporation on transdermal iontophoretic delivery of luteinizing hormone releasing hormone (LHRH) in vitro. Pharm. Res. 1994;11:1809–1814. doi: 10.1023/a:1018983804635. [DOI] [PubMed] [Google Scholar]
- 72.Chang S.L., Hofmann G.A., Zhang L., Deftos L.J., Banga A.K. The effect of electroporation on iontophoretic transdermal delivery of calcium regulating hormones. J. Control. Release. 2000;66:127–133. doi: 10.1016/s0168-3659(99)00262-x. [DOI] [PubMed] [Google Scholar]
- 73.Prausnitz M.R., Edelman E.R., Gimm J.A., Langer R., Weaver J.C. Transdermal delivery of heparin by skin electroporation. Biotechnology. 1995;13:1205–1209. doi: 10.1038/nbt1195-1205. [DOI] [PubMed] [Google Scholar]
- 74.Hooper J.W., Golden J.W., Ferro A.M., King A.D. Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge. Vaccine. 2007;25:1814–1823. doi: 10.1016/j.vaccine.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rosati M., Valentin A., Jalah R., Patel V., von Gegerfelt A., Bergamaschi C., Alicea C., Weiss D., Treece J., Pal R., Markham P.D., Marques E.T., August J.T., Khan A., Draghia-Akli R., Felber B.K., Pavlakis G.N. Increased immune responses in rhesus macaques by DNA vaccination combined with electroporation. Vaccine. 2008;26:5223–5229. doi: 10.1016/j.vaccine.2008.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shen X., Soderholm J., Lin F., Kobinger G., Bello A., Gregg D.A., Broderick K.E., Sardesai N.Y. Influenza A vaccines using linear expression cassettes delivered via electroporation afford full protection against challenge in a mouse model. Vaccine. 2012;30:6946–6954. doi: 10.1016/j.vaccine.2012.02.071. [DOI] [PubMed] [Google Scholar]
- 77.Zorec B., Becker S., Rebersek M., Miklavcic D., Pavselj N. Skin electroporation for transdermal drug delivery: The influence of the order of different square wave electric pulses. Int. J. Pharm. 2013;457:214–223. doi: 10.1016/j.ijpharm.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 78.Kalia Y.N., Naik A., Garrison J., Guy R.H. Iontophoretic drug delivery. Adv. Drug Deliv. Rev. 2004;56:619–658. doi: 10.1016/j.addr.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 79.Marro D., Kalia Y.N., Delgado-Charro M.B., Guy R.H. Contributions of electromigration and electroosmosis to iontophoretic drug delivery. Pharm. Res. 2001;18:1701–1708. doi: 10.1023/a:1013318412527. [DOI] [PubMed] [Google Scholar]
- 80.Marro D., Kalia Y.N., Delgado-Charro M.B., Guy R.H. Optimizing iontophoretic drug delivery: Identification and distribution of the charge-carrying species. Pharm. Res. 2001;18:1709–1713. doi: 10.1023/a:1013370529366. [DOI] [PubMed] [Google Scholar]
- 81.Guy R.H., Kalia Y.N., Delgado-Charro M.B., Merino V., Lopez A., Marro D. Iontophoresis: Electrorepulsion and electroosmosis. J. Control. Release. 2000;64:129–132. doi: 10.1016/s0168-3659(99)00132-7. [DOI] [PubMed] [Google Scholar]
- 82.Gratieri T., Kalia Y.N. Targeted local simultaneous iontophoresis of chemotherapeutics for topical therapy of head and neck cancers. Int. J. Pharm. 2014;460:24–27. doi: 10.1016/j.ijpharm.2013.10.053. [DOI] [PubMed] [Google Scholar]
- 83.Abla N., Naik A., Guy R.H., Kalia Y.N. Contributions of electromigration and electroosmosis to peptide iontophoresis across intact and impaired skin. J. Control. Release. 2005;108:319–330. doi: 10.1016/j.jconrel.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 84.Azagury A., Khoury L., Enden G., Kost J. Ultrasound mediated transdermal drug delivery. Adv. Drug Deliv. Rev. 2014;72:127–143. doi: 10.1016/j.addr.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 85.Polat B.E., Hart D., Langer R., Blankschtein D. Ultrasound-mediated transdermal drug delivery: Mechanisms, scope, and emerging trends. J. Control. Release. 2011;152:330–348. doi: 10.1016/j.jconrel.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krasovitski B., Frenkel V., Shoham S., Kimmel E. Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects. Proc. Natl. Acad. Sci. USA. 2011;108:3258–3263. doi: 10.1073/pnas.1015771108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Benson H.A.E., McElnay J.C., Harland R. Phonophoresis of lignocaine and prilocaine from Emla cream. Int. J. Pharm. 1988;44:65–69. [Google Scholar]
- 88.McElnay J.C., Benson H.A.E., Harland R., Hadgraft J. Phonophoresis of methyl nicotinate: A preliminary study to elucidate the mechanism of action. Pharm. Res. 1993;10:1726–1731. doi: 10.1023/a:1018918013489. [DOI] [PubMed] [Google Scholar]
- 89.Benson H.A.E., McElnay J.C. Transmission of ultrasound energy through topical pharmaceutical products. Physiotherapy. 1988;74:587–589. [Google Scholar]
- 90.Mitragotri S., Kost J. Low-frequency sonophoresis: A review. Adv. Drug Deliv. Rev. 2004;56:589–601. doi: 10.1016/j.addr.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 91.Schoellhammer C.M., Polat B.E., Mendenhall J., Maa R., Jones B., Hart D.P., Langer R., Blankschtein D. Rapid skin permeabilization by the simultaneous application of dual-frequency, high-intensity ultrasound. J. Control. Release. 2012;163:154–160. doi: 10.1016/j.jconrel.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park E.J., Werner J., Smith N.B. Ultrasound mediated transdermal insulin delivery in pigs using a lightweight transducer. Pharm. Res. 2007;24:1396–1401. doi: 10.1007/s11095-007-9306-4. [DOI] [PubMed] [Google Scholar]
- 93.Doukas A.G., Kollias N. Transdermal drug delivery with a pressure wave. Adv. Drug Deliv. Rev. 2004;56:559–579. doi: 10.1016/j.addr.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 94.Murthy S.N., Sammeta S.M., Bowers C. Magnetophoresis for enhancing transdermal drug delivery: Mechanistic studies and patch design. J. Control. Release. 2010;148:197–203. doi: 10.1016/j.jconrel.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krishnan G., Edwards J., Chen Y., Benson H.A.E. Enhanced skin permeation of naltrexone by pulsed electromagnetic fields in human skin in vitro. J. Pharm. Sci. 2010;99:2724–2731. doi: 10.1002/jps.22024. [DOI] [PubMed] [Google Scholar]
- 96.Vicenzino B., Lawrenson P., Khan A., Stephenson A., Heales L., Benson H.A.E. Wright. A. A randomised pilot equivalence trial to evaluate 1 diamagnetically enhanced transdermal delivery of key ground substance components in comparison to an established transdermal non-steroidal anti-inflammatory formulation in males with prior knee injury. PLoS One. 2019 doi: 10.1371/journal.pone.0211999. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mohammed Y.H., Yamada M., Lin L.L., Grice J.E., Roberts M.S., Raphael A.P., Benson H.A., Prow T.W. Microneedle enhanced delivery of cosmeceutically relevant peptides in human skin. PLoS One. 2014;9:e101956. doi: 10.1371/journal.pone.0101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Karimipour D.J., Karimipour G., Orringer J.S. Microdermabrasion: An evidence-based review. Plast. Reconstr. Surg. 2010;125:372–377. doi: 10.1097/PRS.0b013e3181c2a583. [DOI] [PubMed] [Google Scholar]
- 99.Andrews S.N., Zarnitsyn V., Bondy B., Prausnitz M.R. Optimization of microdermabrasion for controlled removal of stratum corneum. Int. J. Pharm. 2011;407:95–104. doi: 10.1016/j.ijpharm.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Andrews S., Lee J.W., Prausnitz M. Recovery of skin barrier after stratum corneum removal by microdermabrasion. AAPS PharmSciTech. 2011;12:1393–1400. doi: 10.1208/s12249-011-9715-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou Y., Banga A.K. Enhanced delivery of cosmeceuticals by microdermabrasion. J. Cosmet. Dermatol. 2011;10:179–184. doi: 10.1111/j.1473-2165.2011.00565.x. [DOI] [PubMed] [Google Scholar]
- 102.Lee W.R., Tsai R.Y., Fang C.L., Liu C.J., Hu C.H., Fang J.Y. Microdermabrasion as a novel tool to enhance drug delivery via the skin: An animal study. Dermatol. Surg. 2006;32:1013–1022. doi: 10.1111/j.1524-4725.2006.32224.x. [DOI] [PubMed] [Google Scholar]
- 103.Andrews S., Lee J.W., Choi S.O., Prausnitz M.R. Transdermal insulin delivery using microdermabrasion. Pharm. Res. 2011;28:2110–2118. doi: 10.1007/s11095-011-0435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Linkner R.V., Jim On S., Haddican M., Singer G., Shim-Chang H. Evaluating the efficacy of photodynamic therapy with 20% aminolevulinic acid and microdermabrasion as a combination treatment regimen for acne scarring: A split-face, randomized, double-blind pilot study. J. Clin. Aesthet. Dermatol. 2014;7:32–35. [PMC free article] [PubMed] [Google Scholar]
- 105.Lee J.W., Gadiraju P., Park J.H., Allen M.G., Prausnitz M.R. Microsecond thermal ablation of skin for transdermal drug delivery. J. Control. Release. 2011;154:58–68. doi: 10.1016/j.jconrel.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bramson J., Dayball K., Evelegh C., Wan Y.H., Page D., Smith A. Enabling topical immunization via microporation: A novel method for pain-free and needle-free delivery of adenovirus-based vaccines. Gene Ther. 2003;10:251–260. doi: 10.1038/sj.gt.3301886. [DOI] [PubMed] [Google Scholar]
- 107.Sintov A.C., Krymberk I., Daniel D., Hannan T., Sohn Z., Levin G. Radiofrequency-driven skin microchanneling as a new way for electrically assisted transdermal delivery of hydrophilic drugs. J. Control. Release. 2003;89:311–320. doi: 10.1016/s0168-3659(03)00123-8. [DOI] [PubMed] [Google Scholar]
- 108.Levin G., Gershonowitz A., Sacks H., Stern M., Sherman A., Rudaev S., Zivin I., Phillip M. Transdermal delivery of human growth hormone through RF-microchannels. Pharm. Res. 2005;22:550–555. doi: 10.1007/s11095-005-2498-6. [DOI] [PubMed] [Google Scholar]
- 109.Lin C.H., Aljuffali I.A., Fang J.Y. Lasers as an approach for promoting drug delivery via skin. Expert Opin. Drug Deliv. 2014;11:599–614. doi: 10.1517/17425247.2014.885501. [DOI] [PubMed] [Google Scholar]
- 110.Scheiblhofer S., Strobl A., Hoepflinger V., Thalhamer T., Steiner M., Thalhamer J., Weiss R. Skin vaccination via fractional infrared laser ablation - Optimization of laser-parameters and adjuvantation. Vaccine. 2017;35:1802–1809. doi: 10.1016/j.vaccine.2016.11.105. [DOI] [PubMed] [Google Scholar]
- 111.Scheiblhofer S., Thalhamer J., Weiss R. Laser microporation of the skin: Prospects for painless application of protective and therapeutic vaccines. Expert Opin. Drug Deliv. 2013;10:761–773. doi: 10.1517/17425247.2013.773970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rkein A., Ozog D., Waibel J.S. Treatment of atrophic scars with fractionated CO2 laser facilitating delivery of topically applied poly-L-lactic acid. Dermatol. Surg. 2014;40:624–631. doi: 10.1111/dsu.0000000000000010. [DOI] [PubMed] [Google Scholar]
- 113.Lee W.R., Shen S.C., Aljuffali I.A., Li Y.C., Fang J.Y. Impact of different vehicles for laser-assisted drug permeation via skin: Full-surface versus fractional ablation. Pharm. Res. 2014;31:382–393. doi: 10.1007/s11095-013-1167-4. [DOI] [PubMed] [Google Scholar]
- 114.Lee W.R., Shen S.C., Chen W.Y., Aljuffali I.A., Suen S.Y., Fang J.Y. Noninvasive delivery of siRNA and plasmid DNA into skin by fractional ablation: Erbium:YAG laser versus CO2 laser. Eur. J. Pharm. Biopharm. 2014;86:315–323. doi: 10.1016/j.ejpb.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 115.Mitragotri S. Current status and future prospects of needle-free liquid jet injectors. Nat. Rev. Drug Discov. 2006;5:543–548. doi: 10.1038/nrd2076. [DOI] [PubMed] [Google Scholar]
- 116.Stachowiak J.C., Li T.H., Arora A., Mitragotri S., Fletcher D.A. Dynamic control of needle-free jet injection. J. Control. Release. 2009;135:104–112. doi: 10.1016/j.jconrel.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 117.Park M.A., Jang H.J., Sirotkin F.V., Yoh J.J. Er:YAG laser pulse for small-dose splashback-free microjet transdermal drug delivery. Opt. Lett. 2012;37:3894–3896. doi: 10.1364/ol.37.003894. [DOI] [PubMed] [Google Scholar]
- 118.Jang H.J., Yu H., Lee S., Hur E., Kim Y., Lee S.H., Kang N., Yoh J.J. Towards clinical use of a laser-induced microjet system aimed at reliable and safe drug delivery. J. Biomed. Opt. 2014;19:058001. doi: 10.1117/1.JBO.19.5.058001. [DOI] [PubMed] [Google Scholar]
- 119.Jang H.J., Hur E., Kim Y., Lee S.H., Kang N.G., Yoh J.J. Laser-induced microjet injection into preablated skin for more effective transdermal drug delivery. J. Biomed. Opt. 2014;19:118002. doi: 10.1117/1.JBO.19.11.118002. [DOI] [PubMed] [Google Scholar]
- 120.Jang H.J., Yeo S., Yoh J.J. Skin pre-ablation and laser assisted microjet injection for deep tissue penetration. Lasers Surg. Med. 2017;49:387–394. doi: 10.1002/lsm.22608. [DOI] [PubMed] [Google Scholar]
- 121.Zilony N., Tzur-Balter A., Segal E., Shefi O. Bombarding cancer: Biolistic delivery of therapeutics using porous Si carriers. Sci. Rep. 2013;3:2499. doi: 10.1038/srep02499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bergmann-Leitner E.S., Leitner W.W. Improving DNA vaccines against malaria: Could immunization by gene gun be the answer? Ther. Deliv. 2013;4:767–770. doi: 10.4155/tde.13.48. [DOI] [PubMed] [Google Scholar]
- 123.Wang S., Zhang C., Zhang L., Li J., Huang Z., Lu S. The relative immunogenicity of DNA vaccines delivered by the intramuscular needle injection, electroporation and gene gun methods. Vaccine. 2008;26:2100–2110. doi: 10.1016/j.vaccine.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bragstad K., Martel C.J., Thomsen J.S., Jensen K.L., Nielsen L.P., Aasted B., Fomsgaard A. Pandemic influenza 1918 H1N1 and 1968 H3N2 DNA vaccines induce cross-reactive immunity in ferrets against infection with viruses drifted for decades. Influenza Other Respir. Viruses. 2011;5:13–23. doi: 10.1111/j.1750-2659.2010.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu Y., Kendall M.A. Optimization of a jet-propelled particle injection system for the uniform transdermal delivery of drug/vaccine. Biotechnol. Bioeng. 2007;97:1300–1308. doi: 10.1002/bit.21324. [DOI] [PubMed] [Google Scholar]
- 126.Quinn H.L., Kearney M.C., Courtenay A.J., McCrudden M.T., Donnelly R.F. The role of microneedles for drug and vaccine delivery. Expert Opin. Drug Deliv. 2014;11:1769–1780. doi: 10.1517/17425247.2014.938635. [DOI] [PubMed] [Google Scholar]
- 127.Kim Y.C., Park J.H., Prausnitz M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012;64:1547–1568. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gill H.S., Denson D.D., Burris B.A., Prausnitz M.R. Effect of microneedle design on pain in human volunteers. Clin. J. Pain. 2008;24:585–594. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Indermun S., Luttge R., Choonara Y.E., Kumar P., du Toit L.C., Modi G., Pillay V. Current advances in the fabrication of microneedles for transdermal delivery. J. Control. Release. 2014;185:130–138. doi: 10.1016/j.jconrel.2014.04.052. [DOI] [PubMed] [Google Scholar]
- 130.Gupta J., Gill H.S., Andrews S.N., Prausnitz M.R. Kinetics of skin resealing after insertion of microneedles in human subjects. J. Control. Release. 2011;154:148–155. doi: 10.1016/j.jconrel.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gill H.S., Prausnitz M.R. Coated microneedles for transdermal delivery. J. Control. Release. 2007;117:227–237. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Choi H.J., Yoo D.G., Bondy B.J., Quan F.S., Compans R.W., Kang S.M., Prausnitz M.R. Stability of influenza vaccine coated onto microneedles. Biomaterials. 2012;33:3756–3769. doi: 10.1016/j.biomaterials.2012.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vrdoljak A., McGrath M.G., Carey J.B., Draper S.J., Hill A.V., O’Mahony C., Crean A.M., Moore A.C. Coated microneedle arrays for transcutaneous delivery of live virus vaccines. J. Control. Release. 2012;159:34–42. doi: 10.1016/j.jconrel.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhu Q., Zarnitsyn V.G., Ye L., Wen Z., Gao Y., Pan L., Skountzou I., Gill H.S., Prausnitz M.R., Yang C., Compans R.W. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc. Natl. Acad. Sci. USA. 2009;106:7968–7973. doi: 10.1073/pnas.0812652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Du G., Woythe L., van der Maaden K., Leone M., Romeijn S., Kros A., Kersten G., Jiskoot W., Bouwstra J.A. Coated and hollow microneedle-mediated intradermal immunization in mice with diphtheria toxoid loaded mesoporous silica nanoparticles. Pharm. Res. 2018;35:189. doi: 10.1007/s11095-018-2476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang H.W., Ye L., Guo X.D., Yang C., Compans R.W., Prausnitz M.R. Ebola vaccination using a DNA vaccine coated on PLGA-PLL/gammaPGA nanoparticles administered using a microneedle patch. Adv. Healthc. Mater. 2017;6 doi: 10.1002/adhm.201600750. [DOI] [PubMed] [Google Scholar]
- 137.Dangol M., Kim S., Li C.G., Fakhraei Lahiji S., Jang M., Ma Y., Huh I., Jung H. Anti-obesity effect of a novel caffeine-loaded dissolving microneedle patch in high-fat diet-induced obese C57BL/6J mice. J. Control. Release. 2017;265:41–47. doi: 10.1016/j.jconrel.2017.03.400. [DOI] [PubMed] [Google Scholar]
- 138.Zhao X., Li X., Zhang P., Du J., Wang Y. Tip-loaded fast-dissolving microneedle patches for photodynamic therapy of subcutaneous tumor. J. Control. Release. 2018;286:201–209. doi: 10.1016/j.jconrel.2018.07.038. [DOI] [PubMed] [Google Scholar]
- 139.Lee I.C., Lin W.M., Shu J.C., Tsai S.W., Chen C.H., Tsai M.T. Formulation of two-layer dissolving polymeric microneedle patches for insulin transdermal delivery in diabetic mice. J. Biomed. Mater. Res. A. 2017;105:84–93. doi: 10.1002/jbm.a.35869. [DOI] [PubMed] [Google Scholar]
- 140.Chen X., Wang L., Yu H., Li C., Feng J., Haq F., Khan A., Khan R.U. Preparation, properties and challenges of the microneedles-based insulin delivery system. J. Control. Release. 2018;288:173–188. doi: 10.1016/j.jconrel.2018.08.042. [DOI] [PubMed] [Google Scholar]
- 141.Zhu W., Pewin W., Wang C., Luo Y., Gonzalez G.X., Mohan T., Prausnitz M.R., Wang B.Z. A boosting skin vaccination with dissolving microneedle patch encapsulating M2e vaccine broadens the protective efficacy of conventional influenza vaccines. J. Control. Release. 2017;261:1–9. doi: 10.1016/j.jconrel.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rodgers A.M., Courtenay A.J., Donnelly R.F. Dissolving microneedles for intradermal vaccination: Manufacture, formulation, and stakeholder considerations. Expert Opin. Drug Deliv. 2018;15:1039–1043. doi: 10.1080/17425247.2018.1522301. [DOI] [PubMed] [Google Scholar]
- 143.Leone M., Priester M.I., Romeijn S., Nejadnik M.R., Monkare J., O’Mahony C., Jiskoot W., Kersten G., Bouwstra J.A. Hyaluronan-based dissolving microneedles with high antigen content for intradermal vaccination: Formulation, physicochemical characterization and immunogenicity assessment. Eur. J. Pharm. Biopharm. 2019;134:49–59. doi: 10.1016/j.ejpb.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 144.Jin Q., Chen H.J., Li X., Huang X., Wu Q., He G., Hang T., Yang C., Jiang Z., Li E., Zhang A., Lin Z., Liu F., Xie X. Reduced graphene oxide nanohybrid-assembled microneedles as mini-invasive electrodes for real-time transdermal biosensing. Small. 2019:e1804298. doi: 10.1002/smll.201804298. [DOI] [PubMed] [Google Scholar]
- 145.Martanto W., Moore J.S., Kashlan O., Kamath R., Wang P.M., O’Neal J.M., Prausnitz M.R. Microinfusion using hollow microneedles. Pharm. Res. 2006;23:104–113. doi: 10.1007/s11095-005-8498-8. [DOI] [PubMed] [Google Scholar]
- 146.Donnelly R.F., Mooney K., McCrudden M.T., Vicente-Perez E.M., Belaid L., Gonzalez-Vazquez P., McElnay J.C., Woolfson A.D. Hydrogel-forming microneedles increase in volume during swelling in skin, but skin barrier function recovery is unaffected. J. Pharm. Sci. 2014;103:1478–1486. doi: 10.1002/jps.23921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Donnelly R.F., McCrudden M.T., Zaid Alkilani A., Larraneta E., McAlister E., Courtenay A.J., Kearney M.C., Singh T.R., McCarthy H.O., Kett V.L., Caffarel-Salvador E., Al-Zahrani S., Woolfson A.D. Hydrogel-forming microneedles prepared from “super swelling” polymers combined with lyophilised wafers for transdermal drug delivery. PLoS One. 2014;9:e111547. doi: 10.1371/journal.pone.0111547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Seong K.Y., Seo M.S., Hwang D.Y., O’Cearbhaill E.D., Sreenan S., Karp J.M., Yang S.Y. A self-adherent, bullet-shaped microneedle patch for controlled transdermal delivery of insulin. J. Control. Release: Off. J. Control. Release. 2017;265:48–56. doi: 10.1016/j.jconrel.2017.03.041. [DOI] [PubMed] [Google Scholar]
- 149.Donnelly R.F., Mooney K., Caffarel-Salvador E., Torrisi B.M., Eltayib E., McElnay J.C. Microneedle-mediated minimally invasive patient monitoring. Ther. Drug Monit. 2014;36:10–17. doi: 10.1097/FTD.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 150.Chang H., Zheng M., Yu X., Than A., Seeni R.Z., Kang R., Tian J., Khanh D.P., Liu L., Chen P., Xu C. A swellable microneedle patch to rapidly extract skin interstitial fluid for timely metabolic analysis. Adv. Mater. 2017;29:201702243. doi: 10.1002/adma. [DOI] [PubMed] [Google Scholar]
- 151.Prausnitz M.R. Engineering Microneedle Patches for Vaccination and Drug Delivery to Skin. Annu. Rev. Chem. Biomol. Eng. 2017;8:177–200. doi: 10.1146/annurev-chembioeng-060816-101514. [DOI] [PubMed] [Google Scholar]
- 152.Ye Y., Yu J., Wen D., Kahkoska A.R., Gu Z. Polymeric microneedles for transdermal protein delivery. Adv. Drug Deliv. Rev. 2018;127:106–118. doi: 10.1016/j.addr.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nguyen T.T., Park J.H. Human studies with microneedles for evaluation of their efficacy and safety. Expert Opin. Drug Deliv. 2018;15:235–245. doi: 10.1080/17425247.2018.1410138. [DOI] [PubMed] [Google Scholar]
- 154.Arya J., Henry S., Kalluri H., McAllister D.V., Pewin W.P., Prausnitz M.R. Tolerability, usability and acceptability of dissolving microneedle patch administration in human subjects. Biomaterials. 2017;128:1–7. doi: 10.1016/j.biomaterials.2017.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Donnelly R.F., Moffatt K., Alkilani A.Z., Vicente-Perez E.M., Barry J., McCrudden M.T., Woolfson A.D. Hydrogel-forming microneedle arrays can be effectively inserted in skin by self-application: A pilot study centred on pharmacist intervention and a patient information leaflet. Pharm. Res. 2014;31:1989–1999. doi: 10.1007/s11095-014-1301-y. [DOI] [PubMed] [Google Scholar]
- 156.Moffatt K., Wang Y., Raj Singh T.R., Donnelly R.F. Microneedles for enhanced transdermal and intraocular drug delivery. Curr. Opin. Pharmacol. 2017;36:14–21. doi: 10.1016/j.coph.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 157.Jin X., Zhu D.D., Chen B.Z., Ashfaq M., Guo X.D. Insulin delivery systems combined with microneedle technology. Adv. Drug Deliv. Rev. 2018;127:119–137. doi: 10.1016/j.addr.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 158.Raphael A.P., Primiero C.A., Ansaldo A.B., Keates H.L., Soyer H.P., Prow T.W. Elongate microparticles for enhanced drug delivery to ex vivo and in vivo pig skin. J. Control. Release. 2013;172:96–104. doi: 10.1016/j.jconrel.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 159.Raphael A.P., Primiero C.A., Lin L.L., Smith R.F., Dyer P., Soyer H.P., Prow T.W. High aspect ratio elongated microparticles for enhanced topical drug delivery in human volunteers. Adv. Healthc. Mater. 2014;3:860–866. doi: 10.1002/adhm.201300517. [DOI] [PubMed] [Google Scholar]
- 160.Yamada M., Tayeb H., Wang H., Dang N., Mohammed Y.H., Osseiran S., Belt P.J., Roberts M.S., Evans C.L., Sainsbury F., Prow T.W. Using elongated microparticles to enhance tailorable nanoemulsion delivery in excised human skin and volunteers. J. Control. Release. 2018;288:264–276. doi: 10.1016/j.jconrel.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Prow T.W., Mohammed Y.H., Ansaldo A.B., Benson H.A.E. Topical microneedle drug delivery enhanced with magnetophoresis. In: Chilcott R.P., Brain K.R., editors. Advances in Dermatological Sciences. RSC Publishing; 2013. pp. 169–177. [Google Scholar]
- 162.Donnelly R.F., Garland M.J., Alkilani A.Z. Microneedle-iontophoresis combinations for enhanced transdermal drug delivery. Methods Mol. Biol. 2014;1141:121–132. doi: 10.1007/978-1-4939-0363-4_7. [DOI] [PubMed] [Google Scholar]
- 163.Dragicevic N., Maibach H. Combined use of nanocarriers and physical methods for percutaneous penetration enhancement. Adv. Drug Deliv. Rev. 2018;127:58–84. doi: 10.1016/j.addr.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 164.Hickling J.K., Jones K.R., Friede M., Zehrung D., Chen D., Kristensen D. Intradermal delivery of vaccines: Potential benefits and current challenges. Bull. World Health Organ. 2011;89:221–226. doi: 10.2471/BLT.10.079426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kupper T.S., Fuhlbrigge R.C. Immune surveillance in the skin: Mechanisms and clinical consequences. Nat. Rev. Immunol. 2004;4:211–222. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Levin C., Perrin H., Combadiere B. Tailored immunity by skin antigen-presenting cells. Hum. Vaccin. Immunother. 2015;11:27–36. doi: 10.4161/hv.34299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.McNeilly C.L., Crichton M.L., Primiero C.A., Frazer I.H., Roberts M.S., Kendall M.A. Microprojection arrays to immunise at mucosal surfaces. J. Control. Release. 2014;196:252–260. doi: 10.1016/j.jconrel.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 168.Rouphael N.G., Paine M., Mosley R., Henry S., McAllister D.V., Kalluri H., Pewin W., Frew P.M., Yu T., Thornburg N.J., Kabbani S., Lai L., Vassilieva E.V., Skountzou I., Compans R.W., Mulligan M.J., Prausnitz M.R. Group, T.-M.S. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): A randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet. 2017;390:649–658. doi: 10.1016/S0140-6736(17)30575-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.McAllister L., Anderson J., Werth K., Cho I., Copeland K., Le Cam Bouveret N., Plant D., Mendelman P.M., Cobb D.K. Needle-free jet injection for administration of influenza vaccine: A randomised non-inferiority trial. Lancet. 2014;384:674–681. doi: 10.1016/S0140-6736(14)60524-9. [DOI] [PubMed] [Google Scholar]
- 170. Needle-free jet injection in workplace influence climes. http://www.ondrugdelivery.com/publications/75/PharmaJet.pdf accessed 11 January 2009.
- 171.Yousafzai M.T., Saleem A.F., Mach O., Baig A., Sutter R.W., Zaidi A.K.M. Feasibility of conducting intradermal vaccination campaign with inactivated poliovirus vaccine using Tropis intradermal needle free injection system, Karachi, Pakistan. Heliyon. 2017;3:e00395. doi: 10.1016/j.heliyon.2017.e00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kim Y.C., Song J.M., Lipatov A.S., Choi S.O., Lee J.W., Donis R.O., Compans R.W., Kang S.M., Prausnitz M.R. Increased immunogenicity of avian influenza DNA vaccine delivered to the skin using a microneedle patch. Eur. J. Pharm. Biopharm. 2012;81:239–247. doi: 10.1016/j.ejpb.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vassilieva E.V., Kalluri H., McAllister D., Taherbhai M.T., Esser E.S., Pewin W.P., Pulit-Penaloza J.A., Prausnitz M.R., Compans R.W., Skountzou I. Improved immunogenicity of individual influenza vaccine components delivered with a novel dissolving microneedle patch stable at room temperature. Drug Deliv. Transl. Res. 2015;5:360–371. doi: 10.1007/s13346-015-0228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wei J.C.J., Haridass I.N., Crichton M.L., Mohammed Y.H., Meliga S.C., Sanchez W.Y., Grice J.E., Benson H.A.E., Roberts M.S., Kendall M.A.F. Space- and time-resolved investigation on diffusion kinetics of human skin following macromolecule delivery by microneedle arrays. Sci. Rep. 2018;8:17759. doi: 10.1038/s41598-018-36009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Fernando G.J., Chen X., Primiero C.A., Yukiko S.R., Fairmaid E.J., Corbett H.J., Frazer I.H., Brown L.E., Kendall M.A. Nanopatch targeted delivery of both antigen and adjuvant to skin synergistically drives enhanced antibody responses. J. Control. Release. 2012;159:215–221. doi: 10.1016/j.jconrel.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 176.Fernando G.J., Zhang J., Ng H.I., Haigh O.L., Yukiko S.R., Kendall M.A. Influenza nucleoprotein DNA vaccination by a skin targeted, dry coated, densely packed microprojection array (Nanopatch) induces potent antibody and CD8(+) T cell responses. J. Control. Release. 2016;237:35–41. doi: 10.1016/j.jconrel.2016.06.045. [DOI] [PubMed] [Google Scholar]
- 177.Chen X., Kask A.S., Crichton M.L., McNeilly C., Yukiko S., Dong L., Marshak J.O., Jarrahian C., Fernando G.J., Chen D., Koelle D.M., Kendall M.A. Improved DNA vaccination by skin-targeted delivery using dry-coated densely-packed microprojection arrays. J. Control. Release. 2010;148:327–333. doi: 10.1016/j.jconrel.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 178.Corbett H.J., Fernando G.J., Chen X., Frazer I.H., Kendall M.A. Skin vaccination against cervical cancer associated human papillomavirus with a novel micro-projection array in a mouse model. PLoS One. 2010;5:e13460. doi: 10.1371/journal.pone.0013460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Muller D.A., Fernando G.J.P., Owens N.S., Agyei-Yeboah C., Wei J.C.J., Depelsenaire A.C.I., Forster A., Fahey P., Weldon W.C., Oberste M.S., Young P.R., Kendall M.A.F. High-density microprojection array delivery to rat skin of low doses of trivalent inactivated poliovirus vaccine elicits potent neutralising antibody responses. Sci. Rep. 2017;7:12644. doi: 10.1038/s41598-017-13011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Muller D.A., Pearson F.E., Fernando G.J., Agyei-Yeboah C., Owens N.S., Corrie S.R., Crichton M.L., Wei J.C., Weldon W.C., Oberste M.S., Young P.R., Kendall M.A. Inactivated poliovirus type 2 vaccine delivered to rat skin via high density microprojection array elicits potent neutralising antibody responses. Sci. Rep. 2016;6:22094. doi: 10.1038/srep22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Baleeiro R.B., Wiesmuller K.H., Reiter Y., Baude B., Dahne L., Patzelt A., Lademann J., Barbuto J.A., Walden P. Topical vaccination with functionalized particles targeting dendritic cells. J. Invest. Dermatol. 2013;133:1933–1941. doi: 10.1038/jid.2013.79. [DOI] [PubMed] [Google Scholar]
- 182.Gilliam A.C., Kremer I.B., Yoshida Y., Stevens S.R., Tootell E., Teunissen M.B., Hammerberg C., Cooper K.D. The human hair follicle: A reservoir of CD40+ B7-deficient Langerhans cells that repopulate epidermis after UVB exposure. J. Invest. Dermatol. 1998;110:422–427. doi: 10.1046/j.1523-1747.1998.00162.x. [DOI] [PubMed] [Google Scholar]
- 183.Yu J., Zhang Y., Ye Y., DiSanto R., Sun W., Ranson D., Ligler F.S., Buse J.B., Gu Z. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc. Natl. Acad. Sci. USA. 2015;112:8260–8265. doi: 10.1073/pnas.1505405112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lee H., Song C., Hong Y.S., Kim M.S., Cho H.R., Kang T., Shin K., Choi S.H., Hyeon T., Kim D.H. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv. 2017;3:e1601314. doi: 10.1126/sciadv.1601314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lee H., Choi T.K., Lee Y.B., Cho H.R., Ghaffari R., Wang L., Choi H.J., Chung T.D., Lu N., Hyeon T., Choi S.H., Kim D.H. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 2016;11:566–572. doi: 10.1038/nnano.2016.38. [DOI] [PubMed] [Google Scholar]
- 186.Son D., Lee J., Qiao S., Ghaffari R., Kim J., Lee J.E., Song C., Kim S.J., Lee D.J., Jun S.W., Yang S., Park M., Shin J., Do K., Lee M., Kang K., Hwang C.S., Lu N., Hyeon T., Kim D.H. Multifunctional wearable devices for diagnosis and therapy of movement disorders. Nat. Nanotechnol. 2014;9:397–404. doi: 10.1038/nnano.2014.38. [DOI] [PubMed] [Google Scholar]


