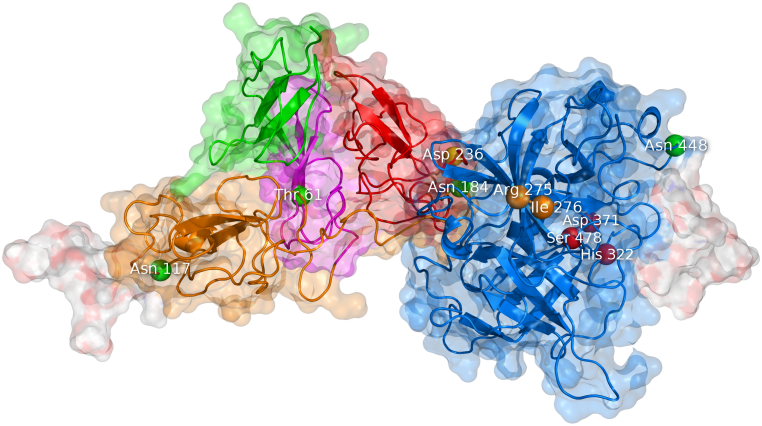
Fig. 6.
Theoretical model of the tertiary structure of tissue plasminogen activator. The visualization is based on the model kindly provided by Ashish and coworkers [78]. T-PA is composed of five domains: finger F (green), epidermal growth factor EGF (purple), kringle K1 (orange), kringle K2 (red), and protease P (blue). The molecule contains four glycosylation sites labeled as green beads: Thr61, Asn117, Asn184, and Asn448. The molecule also has complex oligosaccharides attached to Asn117 and Asn448, which are shown as white surface. The K2 domain contains a lysine binding site at Asp236 (yellow bead). The P domain can be cleaved into two chains in between Arg275 and Ile276 (orange beads). The catalytic triad of the P domain is His322, Asp371, and Ser478 (red beads).