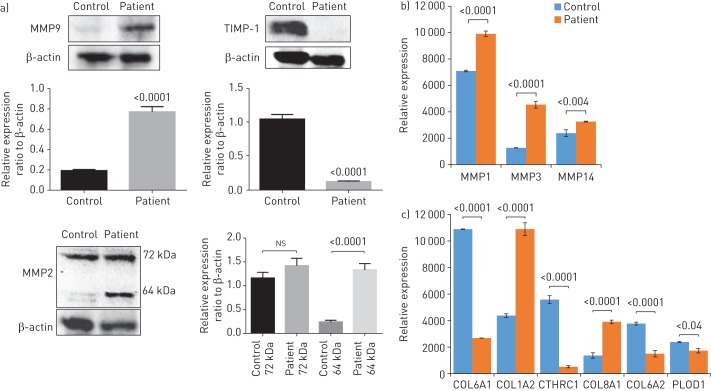
FIGURE 6.
Effect of S100A3 and S100A13 mutations on extracellular matrix (ECM) components. MMP: matrix metalloproteinase; TIMP-1: tissue inhibitor of MMP-1; COL6A1: collagen α-1(VI) chain; COL1A2: collagen α-2(I) chain; CTHRC1: collagen triple helix repeat-containing protein 1; COL8A1: collagen α-1(VIII) chain; COL6A2: collagen α-2(VI) chain; PLOD1: procollagen-lysine, 2-oxoglutarate 5-dioxygenase 1. a) Western blots of MMP2, MMP9 and TIMP-1 expression by skin fibroblasts isolated from healthy controls and patients. Relative expression is depicted in the accompanying histograms. b, c) Differential expression of b) matrixins MMP1, MMP3 and MMP14, and c) ECM-associated proteins COL6A1, COL1A2, CTHRC1, COL8A1, COL6A2 and PLOD1. Normalised protein abundance of significantly differentially expressed proteins between patient and control samples is shown (fold change >1.5 and false discovery rate ∼3%). Yeast alcohol dehydrogenase standard (P00330) at a concentration of 200 fmol per injection was used for “Hi3” absolute quantifications of all identified proteins. The histogram bars correspond to the average protein expression between the two sample groups using the label-free liquid chromatography-mass spectrometry expression analysis system on the Progenesis QI for Proteomics platform. Data are expressed as mean±sem (n=3). p-values are indicated.