Abstract
Skin Langerhans cells are antigen‐presenting cells of the interfollicular epidermis and the upper part of the hair follicle, whereas osteoclasts are specialized bone‐resorbing macrophages. Although at first view these two cell types appear to have little in common, a closer analysis reveals shared features, and when taking into account their surrounding environment, a hypothesis can be developed that Langerhans cells and osteoclasts have evolved from a common ancestral cell type. In this mini‐review, we have compared the ontogenetic features of Langerhans cells and osteoclasts from a genetic and a functional point of view, an issue that so far has been overlooked. The gene programs that control cell differentiation, and the body parts where they reside, present surprising similarities. Whereas the function of osteoclasts in bone degradation has been established since the first vertebrates, Langerhans cells may have undergone a stepwise adaptation from aquatic to terrestrial life. Their cell function co‐evolved with the imperatives of the skin to protect against physical impact, heat, water loss and pathogens, which implied the capacity of Langerhans cells to associate with skin appendages and to develop immunostimulatory functions. For the highly versatile and efficient immune system of modern vertebrates, Langerhans cells may be a memory of the past.
Keywords: Langerhans cell, osteoclast, macrophage, dendritic cell, evolution, hair follicle
Shared ontogenic features of Langerhans cells and osteoclasts
Langerhans cells (LCs) were identified by Paul Langerhans in 1868 when he initially described the cells as cutaneous epithelial nervous cells. A century later they were characterized as antigen‐presenting cells and classified as members of the dendritic cell family. Like dendritic cells, macrophages and B cells, LCs present antigenic peptides associated with the major histocompatibility complex (MHC) I or II to T cells, but – a feature shared only with dendritic cells – they can also migrate from the skin to the lymph nodes to activate native T cells (Maurer & Stingl, 2001). LCs and dendritic cells undergo migration, antigen processing and T‐cell activation in response to innate immune stimuli, such as those triggering Toll‐like receptors, a process called maturation. LCs and dendritic cells therefore associate the innate with the adaptive immune response. LCs are also equipped with CD1 molecules that, like MHC‐I molecules, are composed of an α and a β chain and present lipids to special T cells. Other than antigen presentation, from a functional perspective, LCs seem to have little in common with macrophages, at least what is considered the classical macrophage. However, the definition of macrophage is loose. In fact, macrophages comprise a large family of myeloid cells adapted to their microenvironment. As every tissue contains its own pool of specialized macrophages ‐of embryonic origin or continuously supplied by monocytes – the diversity of these cells is large. It comprises the Kupffer cells of the liver, microglia of the brain, alveolar macrophages of the lung, osteoclasts of the bones, peritoneal macrophages and a heterogeneous population in lymphoid organs. In addition, tissues can also be infiltrated with inflammatory macrophages of monocytic origin and polarized into M1 or M2 types according to the inflammatory program (Gordon & Taylor, 2005). Osteoclasts (OCLs) are macrophages specialized in the degradation of bone. They are characterized by the production of a series of degradative enzymes, acidification of the extracellular space and a directional cell secretory system to channel the localized degradation to bone surface (Cappariello et al. 2014). OCLs function in close association with osteoblasts, cells of the mesenchymal lineage that deposit bone matrix. Active OCLs release factors that promote osteoblast function, leading to a balanced equilibrium between bone resorption and deposition. Animals that lack functional OCLs display osteopetrosis and thus severe growth and developmental defects. Reciprocally, their overactivity leads to osteoporosis. On the other hand, mice deficient in LCs under steady‐state conditions do not show a prominent phenotype (Bennett et al. 2005; Kissenpfennig et al. 2005). This may be attributable to the plasticity of the myeloid lineage and the capacity of monocytes or dermal mononuclear phagocytes to replace LCs under inflammatory conditions.
At first view, LCs and OCLs appear thus very different in form and function, but shared features can be uncovered. OCLs are very large, multinucleated cells formed by the fusion of mononuclear precursor cells. Soft tissue macrophages can also fuse to giant cells such as giant foreign body cells, and dendritic cells can undergo differentiation into OCL‐like cells in response to osteoclastogenic factors (Rivollier et al. 2004; Ibanez et al. 2016). However, whether bona fide LCs fuse into multinucleated cells with OCL function is still debated, although in LC histiocytosis, cells with morphological and antigenic features typical of OCLs have been observed (Egeler et al. 2010). Both LCs and OCLs are part of the myeloid lineage (Gordon, 2003; Satpathy et al. 2012). During embryogenesis, LCs arise from the yolk sac and the fetal liver to colonize the epidermis, where they autorenew throughout life (Hoeffel et al. 2012; Schulz et al. 2012). Under inflammatory conditions, monocytic precursors can differentiate into LCs (Ginhoux et al. 2006). In vitro, like LCs, OCLs can be obtained from primitive macrophages of the yolk sac and the fetal liver (Thesingh, 1986; Kanazawa & Kudo, 2005; however, whether they seed autorenewing OCLs or OCL precursors is not yet known. Surprisingly, OCLs can also process and present antigen to T cells and their T cell‐activating potential differs according to the precursor. Bone marrow monocyte‐derived OCLs induce regulatory T cells, whereas OCLs obtained from dendritic cells promote activation and polarization of T cells into Th1 cells (Ibanez et al. 2016). These findings underline the importance of the type of OCL precursor, which can be monocytes, macrophages or dendritic cells (Jacome‐Galarza et al. 2013).
Mice lacking the transcription factor PU.1 lack all myeloid cells including OCLs and LCs (Tondravi et al. 1997; Chopin et al. 2013). Transforming growth factor (TGF)‐β is required for LC formation and plays a critical role in bone homeostasis by affecting OCL differentiation (Borkowski et al. 1996; Fuller et al. 2000). Both cell types show dependence on signaling by the colony‐stimulating factor 1 receptor (CSF‐1R), as mice deficient in this receptor lack LCs and OCLs (Dai et al. 2002; Ginhoux et al. 2006). In addition, CSF‐1R‐deficient mice are devoid of microglia (Erblich et al. 2011) and show a reduction in the number of monocytes and small intestine Paneth cells (Huynh et al. 2009). The CSF‐1R is activated by CSF‐1 produced by fibroblasts and endothelial cells, and mice with a mutation in the gene coding for CSF‐1 (op/op), rendering it functionally defective, are likewise characterized by reduced OCLs and osteopetrosis (Yoshida et al. 1990). Although LCs depend on CSF‐1R, op/op mice have almost normal numbers of LCs (Witmer‐Pack et al. 1993), and osteopetrosis was more severe in CSF‐1R−/− mice than in op/op mice. Recently, interleukin (IL)‐34 was identified as the alternative ligand for CSF‐1R and was found to support myeloid cell viability and the formation of macrophages from progenitors (Lin et al. 2008). Mice deficient in IL‐34 lack LCs (Wang et al. 2012), demonstrating that LCs require the activation of CSF‐1R by IL‐34. As might be expected, IL‐34 also activates osteoclastogenesis (Nakamichi et al. 2012).
IL‐34 is produced by keratinocytes (Wang et al. 2012), whereas CSF‐1 is principally synthesized by mesenchymal cells and endothelial cells (Ryan et al. 2001). It appears therefore that the association with a neighboring cell type is important in determining cell fate. Indeed, LCs arise only in the epithelium of skin, eye, vagina and the oral surfaces (Young et al. 1985; Sato & Hirano, 1997; Hamrah & Dana, 2007). OCLs on the other hand form in tight association with the osteoblasts, cells of mesenchymal origin and sources of CSF‐1. RANKL (TNFSF11/TRANCE), produced by osteoblasts, is another key cytokine for osteoclastogenesis. Conjointly identified by research groups working on osteoclasts and dendritic cells, RANKL was found to promote the differentiation of myeloid cells into OCLs in the presence of CSF‐1 (Suda et al. 1999). RANKL activates the differentiation into OCLs via the receptor RANK, which signals through different pathways including necrosis factor (NF)‐κB (hence the name receptor activator of NF‐κB) (Anderson et al. 1997). RANKL–RANK interaction is antagonized by a non‐signaling decoy receptor, OPG (osteoprotegerin) (Simonet et al. 1997). Mice deficient in or overexpressing either of the three proteins display alterations in bone density (Walsh & Choi, 2014). This has been confirmed in humans as diseases characterized by osteoclast‐poor forms of osteopetrosis involve loss‐of‐function mutations in RANK or RANKL (OMIM #612301 and OMIM #259710, respectively); inversely, decreased bone density results from defective OPG (OMIM #239000) or hyperactive RANK (OMIM #174810). RANKL and OPG are produced by the osteoblasts, therefore an equilibrium is established whereby under normal conditions bone synthesis by osteoblasts is concomitant with bone matrix resorption by OCLs. Alterations in this equilibrium lead to bone matrix deposition or, conversely, to bone loss. These alterations are triggered by a number of different factors that respond to changes in physical impact (body size, weight, movement), the requirement of calcium and phosphate (hormonal control) or inflammation. However, RANKL not only plays a key role in the regulation of bone mass; it also has an important impact on the immune system. Mice deficient in RANKL lack lymph nodes (Kong et al. 1999), and RANKL stimulates dendritic cell maturation (Anderson et al. 1997). Strikingly, mice deficient in RANKL show a reduction in LC numbers (Barbaroux et al. 2008). LCs express RANK, and RANKL is produced by keratinocytes in the embryo and the newborn mouse (Duheron et al. 2011). Inflammatory conditions can upregulate RANKL expression in the adult (Loser et al. 2006).
In view of these similarities in ontogeny, a valid question is why OCLs are found in the bone and LCs reside in the epidermis. In fact, in the proliferative disorder of Langerhans cell histiocytosis (LCH), cells with Langerhans and osteoclast phenotypic and functional features, occur in osseous sites (calvarian bones, but also femur, ribs and vertebrae) and extraosseous sites (facial and scalp skin, soft tissue of the mouth, lymph nodes, lungs and other internal organs) (Egeler et al. 2010). Also, under in vitro culture conditions and in osteopetrotic mice, dendritic cells can convert to OCLs (Akagawa et al. 1996; Wakkach et al. 2008). Normally, however, cell specialization is confined to distinct body sites, and it is probable that the answer to this question lies in the complex regulatory mechanism imposed by the microenvironment. For instance, GM‐CSF, which is a key cytokine for dendritic cell differentiation but inhibits osteoclastogenesis, is produced by keratinocytes (Miyamoto et al. 2001).
Functional specialization from a common ancestor cell
From an evolutionary standpoint, the current vision is that bone appeared as mineralization of body parts giving rise to teeth and to protective measures, such as shells or spines. This integumentary skeleton (or dermal skeleton, exoskeleton), consists of the dermatocranium, skeletal components of the pectoral apparatus, teeth and tooth‐like elements of the pharyngeal cavities. It provided the animal with advantage of locomotion and protection but made it heavier, limited its size and the development of sensory organs. The delocalization of mineralized material from the outside to the inside of the body facilitated agile locomotion with attachment of muscular systems and the development of the skin as a sensory organ. The skin has therefore undergone a series of adaptations to replace the heavy collagenous – mineralized – materials of the dermal skeleton with the lighter but equally protective keratin proteins of the epidermis, such as reptilian scales. The alternative strategy was to develop skin appendages, fish scales, feathers and hair. These served as physical protection but also could be adapted to allow a lightweight resistance to air for flight. With the adaptation to terrestrial life, the regulation of water loss and the resistance to temperature changes became a major element of survival. Hence, the mucosal surface of fish skin underwent important changes, including keratins, and phospholipids with bound calcium to render the epidermis of terrestians resistant to water loss. The development of sweat glands and hair was necessary to regulate heat exchange. In addition, temperature changes, reproductive seasons and growth phases imposed on the skin the ability to continuously adjust to these forces. As part of its protective program, the epidermis constantly renews and either continuously sheds epidermal layers or undergoes a seasonal shedding process (molting). The skull bones likewise undergo a remodeling program, such as in salmon when migrating from salt water to fresh water or in deer when antlers follow changes of the reproductive seasons. Also the dermal appendages follow phases of renewal. Fish scales are remodeled to fulfill special inorganic material needs (Witten & Huysseune, 2009) and hair can function as a social and sexual character and can adapt to temperatures changes (Randall, 2007).
Genetic programs that oversee endoskeleton formation are active in the skin and in the development of dermal appendages. The Runx, hedgehog or Sox gene families, to name a few, are key gene families that regulate osteoblastogenesis and play a major role in dermal appendage development or renewal (Ross & Christiano, 2006; Wagner & Aspenberg, 2011). RANKL is required for tooth eruption, mammary gland development and hair cycling. Although mammary glands and hair develop normally in the embryo of knock‐out mice, pregnancy‐induced mammary gland growth and the juvenile and adult hair cycling are RANK‐ and RANKL‐dependent (Fata et al. 2000; Duheron et al. 2011). It is intriguing that IL‐34 is required for microglial cells, specialized macrophages of the brain. Fish also contain such microglial cells and there is evidence suggesting that these cells extend into the skin (Herbomel et al. 2001). Myelomonocytic cells of dendritic morphology could also be seen in fish skin using a chemokine receptor reporter transgenic construct expressed in LCs of higher vertebrates (Aghaallaei et al. 2010). This suggests that fish contain LC‐like cells. In chicken and probably also in reptiles (turtle), epidermal cells with LC phenotypic and functional features have been described (Perez‐Torres et al. 1995; Igyarto et al. 2006). This raises the possibility that bona fide LCs, as characterized in mammals, derive from an evolutionary early ancestor; however, in the absence of a sophisticated immune system of higher vertebrates, LCs of these animals would have fulfilled a different functional need. In view of the role of RANKL in controlling the hair follicle cycle (Duheron et al. 2011), its action on LCs and the requirement of CSF‐1R signaling for LC development, it is tempting to speculate that LCs would play a more important role in the homeostasis of skin than is presently thought. Like OCLs that together with osteoblasts regulate bone mass, LCs may be required together with keratinocytes to confer on the skin a capacity to change. Indeed, it has been proposed that LC may coordinate epidermal renewal by affecting cell division of keratinocytes that are organized in proliferation units comprising at their center a LC (Potten & Allen, 1976). Alternatively, LCs in evolutionary older vertebrates may have exerted more macrophage‐type activity to degrade material to promote epidermal shedding. Interestingly, fish scales are resorbed by mononucleated cells, whereas the endoskeleton is largely resorbed by multinucleated giant OCL‐like cells (Witten & Huysseune, 2009). The hair cycle comprises a catabolic process, called catagen, during which keratinocyte‐comprising hair follicles undergo apoptosis. Also in this process, LCs could play a role in the degradation of cellular material. LCs may be a better choice to eliminate apoptotic cell debris than surrounding macrophages, since LCs are already closely associated with the epithelial cells and do not need to be recruited through chemokines or inflammatory mediators, unlike macrophages dispersed throughout the dermis. Indeed, during catagen, LCs were observed to phagocytose hair follicle melanin (Tobin, 1998).
With the evolution of the immune system from bony fish onwards, cell types became specialized to mount an immune response against pathogens and had to learn to distinguish between self and non‐self. For this, cells were needed to continuously educate potentially autoreactive T cells to ignore self‐material. Among the body surfaces most exposed to pathogens is the skin. In mammals LCs have the capacity to migrate from the skin to secondary lymphoid organs, where the T cells reside, to present to them self‐antigens and maintain self‐tolerance. It is therefore possible that the function of LCs in skin homeostasis was co‐opted by the immune system to reuse degraded cellular material for the education of T cells. LCs developed a migratory ability and acquired the capacity to partially degrade material and to present derived peptides on the major histocompatibility complex to T cells (Fig. 1). Possibly a sign of this acquired function are the CD1 molecules, which present lipids derived from bacteria or from cellular membranes in a similar manner to that of peptides on MHC‐I. The T cells that recognize CD1‐lipid complexes already present in jawed vertebrates and some are resident in the epithelium (Castro et al. 2015).
Figure 1.
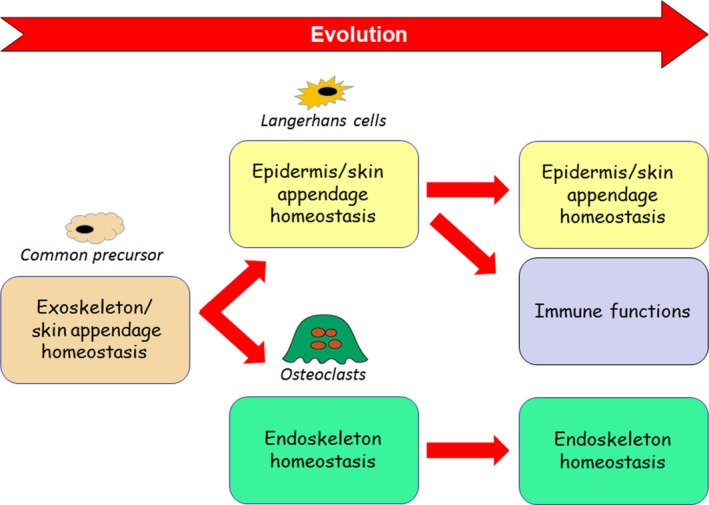
Proposed evolutionary relationship between Langerhans cells and osteoclasts. In early vertebrates a common precursor cell unites the skin‐specialized homeostatic functions of Langerhans cells and the endoskeleton‐specialized homeostatic functions of osteoclasts. Whereas osteoclast functions remain unchanged, Langerhans cells acquire immuno‐regulatory activities.
Taken together, the features shared by LCs and OCLs raise the possibility that an ancestral cell regulating exoskeleton/skin hemostasis of early vertebrates gave rise to skin‐specialized LCs and bone‐specialized OCLs of higher vertebrates. Beyond its general interest in biology, investigations into this proposed model may provide unexpected answers for medically relevant issues such as osteoimmunology, autoimmunity and cancer.
Acknowledgements
The authors declare that there is no conflict of interest. We thank the CNRS for financial support. B.V. was a recipient of a PhD fellowship of the French Ministry of Research and Higher Education and of the Fondation pour la Recherche Médicale (FDT20130928345).
References
- Aghaallaei N, Bajoghli B, Schwarz H, et al. (2010) Characterization of mononuclear phagocytic cells in medaka fish transgenic for a cxcr3a:gfp reporter. Proc Natl Acad Sci U S A 107, 18079–18084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagawa KS, Takasuka N, Nozaki Y, et al. (1996) Generation of CD1+ RelB+ dendritic cells and tartrate‐resistant acid phosphatase‐positive osteoclast‐like multinucleated giant cells from human monocytes. Blood 88, 4029–4039. [PubMed] [Google Scholar]
- Anderson DM, Maraskovsky E, Billingsley WL, et al. (1997) A homologue of the TNF receptor and its ligand enhance T‐cell growth and dendritic‐cell function. Nature 390, 175–179. [DOI] [PubMed] [Google Scholar]
- Barbaroux JB, Beleut M, Brisken C, et al. (2008) Epidermal receptor activator of NF‐kB ligand controls Langerhans cell numbers and proliferation. J Immunol 181, 1103–1108. [DOI] [PubMed] [Google Scholar]
- Bennett CL, van Rijn E, Jung S, et al. (2005) Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol 169, 569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkowski TA, Letterio JJ, Farr AG, et al. (1996) A role for endogenous transforming growth factor beta 1 in Langerhans cell biology: the skin of transforming growth factor beta 1 null mice is devoid of epidermal Langerhans cells. J Exp Med 184, 2417–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappariello A, Maurizi A, Veeriah V, et al. (2014) The Great Beauty of the osteoclast. Arch Biochem Biophys 558, 70–78. [DOI] [PubMed] [Google Scholar]
- Castro CD, Luoma AM, Adams EJ (2015) Coevolution of T‐cell receptors with MHC and non‐MHC ligands. Immunol Rev 267, 30–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopin M, Seillet C, Chevrier S, et al. (2013) Langerhans cells are generated by two distinct PU.1‐dependent transcriptional networks. J Exp Med 210, 2967–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai XM, Ryan GR, Hapel AJ, et al. (2002) Targeted disruption of the mouse colony‐stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood 99, 111–120. [DOI] [PubMed] [Google Scholar]
- Duheron V, Hess E, Duval M, et al. (2011) Receptor Activator of NF‐kB (RANK) stimulates the proliferation of epithelial cells of the epidermo‐pilosebaceous unit. Proc Natl Acad Sci U S A 108, 5342–5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeler RM, van Halteren AG, Hogendoorn PC, et al. (2010) Langerhans cell histiocytosis: fascinating dynamics of the dendritic cell‐macrophage lineage. Immunol Rev 234, 213–232. [DOI] [PubMed] [Google Scholar]
- Erblich B, Zhu L, Etgen AM, et al. (2011) Absence of colony stimulation factor‐1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLoS One 6, e26317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata JE, Kong YY, Li J, et al. (2000) The osteoclast differentiation factor osteoprotegerin‐ligand is essential for mammary gland development. Cell 103, 41–50. [DOI] [PubMed] [Google Scholar]
- Fuller K, Lean JM, Bayley KE, et al. (2000) A role for TGF‐beta 1 in osteoclast differentiation and survival. J Cell Sci 113, 2445–2453. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Tacke F, Angeli V, et al. (2006) Langerhans cells arise from monocytes in vivo . Nat Immunol 7, 265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S (2003) Alternative activation of macrophages. Nat Rev Immunol 3, 23–35. [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor PR (2005) Monocyte and macrophage heterogeneity. Nat Rev Immunol 5, 953–964. [DOI] [PubMed] [Google Scholar]
- Hamrah P, Dana MR (2007) Corneal antigen‐presenting cells. Chem Immunol Allergy 92, 58–70. [DOI] [PubMed] [Google Scholar]
- Herbomel P, Thisse B, Thisse C (2001) Zebrafish early macrophages colonize cephalic mesenchyme and developing brain, retina, and epidermis through a M‐CSF receptor‐dependent invasive process. Dev Biol 238, 274–288. [DOI] [PubMed] [Google Scholar]
- Hoeffel G, Wang Y, Greter M, et al. (2012) Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac‐derived macrophages. J Exp Med 209, 1167–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh D, Dai XM, Nandi S, et al. (2009) Colony stimulating factor‐1 dependence of paneth cell development in the mouse small intestine. Gastroenterology 137, 136–144. e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez L, Abou‐Ezzi G, Ciucci T, et al. (2016) Inflammatory osteoclasts prime TNFα‐producing CD4+ T cells and express CX3 CR1. J Bone Miner Res. (in press) doi: 10.1002/jbmr.28268. [DOI] [PubMed] [Google Scholar]
- Igyarto BZ, Lacko E, Olah I, et al. (2006) Characterization of chicken epidermal dendritic cells. Immunology 119, 278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacome‐Galarza CE, Lee SK, Lorenzo JA, et al. (2013) Identification, characterization, and isolation of a common progenitor for osteoclasts, macrophages, and dendritic cells from murine bone marrow and periphery. J Bone Miner Res 28, 1203–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa K, Kudo A (2005) TRAF2 is essential for TNF‐α‐induced osteoclastogenesis. J Bone Miner Res 20, 840–847. [DOI] [PubMed] [Google Scholar]
- Kissenpfennig A, Henri S, Dubois B, et al. (2005) Dynamics and function of Langerhans cells in vivo dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity 22, 643–654. [DOI] [PubMed] [Google Scholar]
- Kong YY, Yoshida H, Sarosi I, et al. (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph‐node organogenesis. Nature 397, 315–323. [DOI] [PubMed] [Google Scholar]
- Lin H, Lee E, Hestir K, et al. (2008) Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science 320, 807–811. [DOI] [PubMed] [Google Scholar]
- Loser K, Mehling A, Loeser S, et al. (2006) Epidermal RANKL controls regulatory T‐cell numbers via activation of dendritic cells. Nat Med 12, 1372–1379. [DOI] [PubMed] [Google Scholar]
- Maurer D, Stingl G (2001) Langerhans cells In: Dendritic Cells. (eds Lotze MT, Thomson AW.), pp. 35–50, San Diego: Academic Press. [Google Scholar]
- Miyamoto T, Ohneda O, Arai F, et al. (2001) Bifurcation of osteoclasts and dendritic cells from common progenitors. Blood 98, 2544–2554. [DOI] [PubMed] [Google Scholar]
- Nakamichi Y, Mizoguchi T, Arai A, et al. (2012) Spleen serves as a reservoir of osteoclast precursors through vitamin D‐induced IL‐34 expression in osteopetrotic op/op mice. Proc Natl Acad Sci U S A 109, 10006–10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez‐Torres A, Millan‐Aldaco DA, Rondan‐Zarate A (1995) Epidermal Langerhans cells in the terrestrial turtle, Kinosternum integrum . Dev Comp Immunol 19, 225–236. [DOI] [PubMed] [Google Scholar]
- Potten CS, Allen TD (1976) A model implicating the Langerhans cell in keratinocyte proliferation control. Differentiation 5, 43–47. [DOI] [PubMed] [Google Scholar]
- Randall VA (2007) Hormonal regulation of hair follicles exhibits a biological paradox. Semin Cell Dev Biol 18, 274–285. [DOI] [PubMed] [Google Scholar]
- Rivollier A, Mazzorana M, Tebib J, et al. (2004) Immature dendritic cell transdifferentiation into osteoclasts: a novel pathway sustained by the rheumatoid arthritis microenvironment. Blood 104, 4029–4037. [DOI] [PubMed] [Google Scholar]
- Ross FP, Christiano AM (2006) Nothing but skin and bone. J Clin Invest 116, 1140–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan GR, Dai XM, Dominguez MG, et al. (2001) Rescue of the colony‐stimulating factor 1 (CSF‐1)‐nullizygous mouse (Csf1(op)/Csf1(op)) phenotype with a CSF‐1 transgene and identification of sites of local CSF‐1 synthesis. Blood 98, 74–84. [DOI] [PubMed] [Google Scholar]
- Sato K, Hirano M (1997) Langerhans cells in the larynx and the hypopharynx. Kurume Med J 44, 297–303. [DOI] [PubMed] [Google Scholar]
- Satpathy AT, Wu X, Albring JC, et al. (2012) Re(de)fining the dendritic cell lineage. Nat Immunol 13, 1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz C, Gomez Perdiguero E, Chorro L, et al. (2012) A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90. [DOI] [PubMed] [Google Scholar]
- Simonet WS, Lacey DL, Dunstan CR, et al. (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89, 309–319. [DOI] [PubMed] [Google Scholar]
- Suda T, Takahashi N, Udagawa N, et al. (1999) Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev 20, 345–357. [DOI] [PubMed] [Google Scholar]
- Thesingh CW (1986) Formation sites and distribution of osteoclast progenitor cells during the ontogeny of the mouse. Dev Biol 117, 127–134. [DOI] [PubMed] [Google Scholar]
- Tobin DJ (1998) A possible role for Langerhans cells in the removal of melanin from early catagen hair follicles. Br J Dermatol 138, 795–798. [DOI] [PubMed] [Google Scholar]
- Tondravi MM, McKercher SR, Anderson K, et al. (1997) Osteopetrosis in mice lacking haematopoietic transcription factor PU.1. Nature 386, 81–84. [DOI] [PubMed] [Google Scholar]
- Wagner DO, Aspenberg P (2011) Where did bone come from? Acta Orthop 82, 393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakkach A, Mansour A, Dacquin R, et al. (2008) Bone marrow microenvironment controls the in vivo differentiation of murine dendritic cells into osteoclasts. Blood 112, 5074–5083. [DOI] [PubMed] [Google Scholar]
- Walsh MC, Choi Y (2014) Biology of the RANKL‐RANK‐OPG system in immunity, bone, and beyond. Front Immunol 5, 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Szretter KJ, Vermi W, et al. (2012) IL‐34 is a tissue‐restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol 13, 753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witmer‐Pack MD, Hughes DA, Schuler G, et al. (1993) Identification of macrophages and dendritic cells in the osteopetrotic (op/op) mouse. J Cell Sci 104, 1021–1029. [DOI] [PubMed] [Google Scholar]
- Witten PE, Huysseune A (2009) A comparative view on mechanisms and functions of skeletal remodelling in teleost fish, with special emphasis on osteoclasts and their function. Biol Rev 84, 315–346. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Hayashi S, Kunisada T, et al. (1990) The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 345, 442–444. [DOI] [PubMed] [Google Scholar]
- Young WG, Newcomb GM, Hosking AR (1985) The effect of atrophy, hyperplasia, and keratinization accompanying the estrous cycle on Langerhans' cells in mouse vaginal epithelium. Am J Anat 174, 173–186. [DOI] [PubMed] [Google Scholar]


