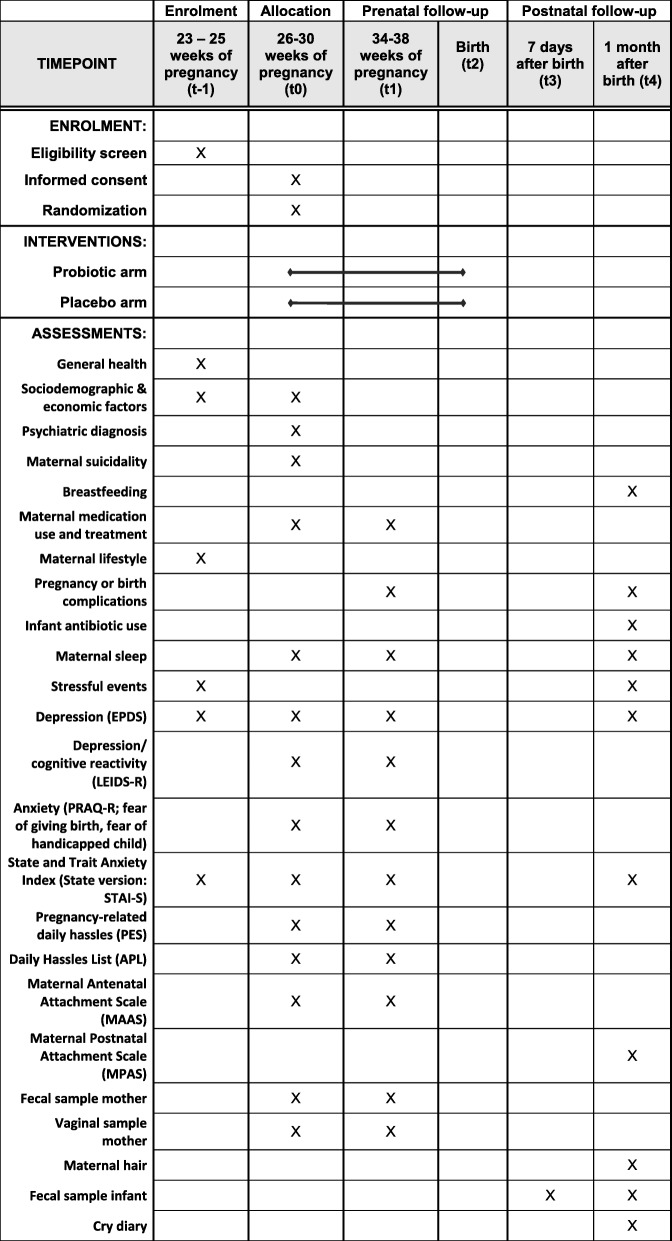
Fig. 1.
Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) figure displaying the pilot trial design and the outcome measurements. After screening for eligibility (t-1), women sign the informed consent form and complete the baseline questionnaire (t0). Participants are then randomized in the probiotic or placebo arm and receive either the probiotic food supplement or placebo. After randomization, filling in the baseline questionnaire and collecting maternal vaginal and stool samples, women start using either the probiotic food supplement or placebo. Measurements take place at 26–30 weeks of pregnancy (baseline(t0); 34–38 weeks of pregnancy (t1); 7 days after birth (t3), and 1 month after birth (t4)).