Abstract
A primary goal of this study was to examine the impact of an Integrated Brain, Body, and Social (IBBS) intervention on ERPs of attentional control (P3 & N2) in children with ADHD. The secondary goal was to test the differences between children with and without ADHD on ERP and Go/No-Go behavioral measures. A total of twenty-nine participants (M age = 7.14 years; 52% male; 41.4% white) recruited from the IBBS efficacy study comparing IBBS to Treatment-As-Usual (TAU) completed a Go/No-Go task before and after treatment as brain activity was recorded using EEG. Thirty-four matched healthy controls (HC) completed the same EEG procedures at a single time point. Following treatment, the Go P3 latency was significantly earlier for the IBBS group relative to the TAU group. No treatment effects were found on any behavioral measures. Prior to treatment, there was a significant difference between the ADHD group and HC group for the N2 difference wave. Children with ADHD also showed slower reaction times on behavioral measures. Although this pilot study did not reveal robust treatment effects, it suggests that IBBS may prevent the worsening of attentional systems in the brain and larger studies are needed for replication purposes.
Keywords: Cognitive Training, Physical Exercise, Behavior Management, Electrophysiology, Randomized Controlled Trial
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a common neurodevelopmental disorder characterized by age-inappropriate levels of inattention, hyperactivity, and impulsivity that causes impaired functioning. These symptoms often persist into adulthood and are associated with poor developmental outcomes including occupational problems, other psychiatric disorders, substance abuse, criminal activity, and higher mortality rates (Cherkasova et al., 2013; Dalsgaard et al., 2015; Knecht et al., 2015). ADHD symptoms are thought to reflect an underlying executive function (EF) deficit, primarily in the domains of attention, response inhibition, and working memory (Castellanos and Tannock, 2002; Hervey et al., 2004). EFs are higher-order cognitive processes underlying goal-directed behavior that have a profound influence on a child’s ability to learn, problem-solve, plan, and perform everyday tasks and activities (Kornell and Metcalfe, 2006). An EF deficit model of ADHD is supported by findings of poorer performance on neuropsychological tests where children with ADHD show more errors and have slower, more variable responses than healthy control (HC) children (Willcutt et al., 2005). However, these group differences are usually medium-sized effects (Cohen’s d = 0.4–0.7), with not all children with ADHD showing EF deficits (Willcutt et al., 2005).
A lack of consistency in finding EF impairments among children with ADHD may be explained in several ways. First, the methods used to measure EFs are widely variable (e.g., multiple versions of the same test, multiple scores extracted from these tests with varying levels of sensitivity; see Seidman, 2006). Second, EFs are extremely difficult to operationalize or capture, as each EF consists of several non-shared components (A.M.C Kelly et al., 2006; Miyake, Friedman, Emerson, Witzki, and Howerter, 2000). Third, it has been suggested that there may be multiple EF profiles subsumed under the broader ADHD diagnostic category (Nigg et al., 2005), where some children show the greatest difficulties with impulsivity related to response inhibition, others struggle with cognitive flexibility in shifting attention between tasks, and still others cannot be differentiated from their typically developing peers based on their neuropsychological profile (Castellanos et al., 2006; Fair, Bathula, Nikolas, and Nigg, 2012; Nigg et al., 2005); subtypes that have been recently supported by emerging evidence from a study employing cluster analyses (Roberts, Martel, and Nigg, 2017). Fourth, neuropsychological tests may not be sensitive enough to detect the EF deficits of all children diagnosed with ADHD, as they tend to reflect the end product of underlying cognitive processes in the form of a behavioral response. The majority of EF measures have adequate predictive validity for ADHD (i.e., true positives); however, an average performance on a neuropsychological test cannot rule out an ADHD diagnosis (i.e., false negatives; Doyle et al., 2000; Hinshaw et al., 2002; Lovejoy et al., 1999). Thus, such tests are not recommended as the only method to establish an ADHD diagnosis, but are useful in providing cognitive profiles of strengths and weaknesses for treatment purposes and assessing changes in functioning over time (Seidman and Bruder, 2003; Seidman and Toomey, 1999).
To better elucidate the neurocognitive processes that increase the risk for ADHD, electrophysiological measures, such as event-related potentials (ERPs), have been paired with neuropsychological tests to detect the cascade of neural processes before, during, and after a behavioral response. Two ERP measures that are relevant to ADHD and typically elicited during Cued Go/No-Go tasks include the P3b (positive deflection occurring at around 300ms after the stimulus at posterior parietal locations) and N2 (negative deflection occurring at around 200ms after the stimulus at fronto-central sites), as they index information processing with respect to stimulus discrimination/updating for task-related improbable events (P3b) and conflict monitoring (N2), respectively, and are both associated with attentional control (Barry et al., 2003; Donkers and van Boxtel, 2004; Helenius et al., 2011; Polich, 2007; Tye et al., 2014). Studies in children with ADHD are consistent in showing attenuated P3 amplitudes during Go and No-Go trials and attenuated N2 amplitudes during No-Go trials as compared to HC children (Albrecht et al., 2008; Barry et al., 2003; Brandeis et al., 2002; Wiersema et al., 2006). Moreover, increased N2 latencies have been reported in young children and adolescents with ADHD (Lazzaro et al., 2001; Satterfield et al., 1984). However, results for P3 latency has been more mixed with some studies finding increased P3 latencies, others finding decreased P3 latencies, and still others finding no differences between ADHD and HC groups (Loiselle et al., 1980; Johnstone et al., 2001; Winsberg et al., 1993). Finally, a reduced or non-existent No-Go effect (No-Go amplitude > Go amplitude) for the P3 and N2 has been revealed in participants with ADHD (Holcomb et al., 1986; Satterfield et al., 1988). A larger No-Go amplitude than Go amplitude is expected as more attentional allocation is required to detect changes triggered by the less frequent No-Go stimuli.
ERPs have also been increasingly used to evaluate treatment response at the neurophysiological level. Stimulant medications (i.e., methylphenidate) have been shown to increase P3 and N2 amplitudes in children with ADHD so they are comparable to HC children (Broyd et al., 2005; Groom et al., 2010; Janssen et al., 2016; Ozdag et al., 2004). Medication effects have also been shown for ERP latencies, with P3 latencies reduced (earlier response to infrequent targets) and N2 latencies enhanced (later response to infrequent targets) following treatment (Ozdag et al., 2004; Sunohara et al., 1999). Interestingly, Broyd and colleagues (2005) found that for younger children with ADHD (aged 8 to 11 years), the Go N2 amplitude was significantly larger than the No-Go N2 amplitude pre-medication and the expected No-Go N2 effect (No-Go N2 > Go N2), as seen in HC children, was found post-medication. Overall, these results suggest that N2 and P3 ERP measures may be sensitive to the effects of ADHD interventions.
This study evaluated the impact of a non-pharmacological treatment for ADHD, an Integrated Brain, Body, and Social (IBBS) intervention, on ERP measures elicited by a Go/No-Go task. The development of new interventions for ADHD is warranted as existing and well-established interventions (e.g., medication, behavior management) do not address all executive function impairments associated with ADHD, which was the primary intent behind developing cognitive working memory training (CWMT) interventions. Although initial findings were quite promising for CWMT as neuropsychological tests of working memory showed improvement (e.g., Bigorra et al., 2015; Johnstone et al., 2012; Klingberg et al., 2005, 2002), recent work suggests that the effects of CWMT may not transfer to untrained domains (i.e., ADHD symptomatology, other related cognitive processes) (Chacko et al., 2014; Gray et al., 2012; Green et al., 2012; Johnstone et al., 2012; Mawjee et al., 2015; Rapport et al., 2013; Smith et al., 2016; Sonuga-Barke et al., 2013; van der Donk et al., 2015; van Dongen-Boomsma et al., 2014) and issues concerning the dose and broadness of these training programs may have contributed to a lack of treatment effects. To address these limitations, IBBS combined computerized cognitive training (brain component) with physical exercise (body component) and an evidence-based behavior management strategy (social component) and built on previous CWMTs in three important ways. First, IBBS trains eight executive functions (i.e., sustained attention, response inhibition, speed of processing, cognitive flexibility, multiple simultaneous attention, working memory, category formation, pattern recognition) known to be implicated in ADHD (Crippa et al., 2015; Huang-Pollock et al., 2014; Willcutt et al., 2005) whereas standard CWMT only targets working memory. Second, IBBS employs an additional method of training by means of physical exercise, as previous research has shown the benefits of exercise in improving performance on neuropsychological tests of EFs (Grassmann et al., 2014; Kamp et al., 2014) and ratings of ADHD symptoms (Abramovitch et al., 2013; Verret et al., 2012). Third, IBBS includes a behavior management technique (social component of IBBS) to encourage the engagement of children as they completed cognitive training and physical exercises (Chacko et al., 2014). Importantly, previous research has suggested that CWMT and acute physical exercise improve ERPs of attentional control among children with ADHD (Johnstone et al., 2010; Ludyga et al., 2017; Pontifex et al., 2013). Specifically, CWMT has resulted in increased N2 amplitudes, particularly for No-Go trials, following 5-weeks of cognitive training, and a one-time session of acute exercise has led to larger P3 amplitudes post-treatment.
The results of a randomized controlled trial comparing IBBS versus Treatment-As-Usual (TAU) and its impact on ADHD symptomatology and neurocognitive functioning are presented elsewhere (Smith et al., 2016) and this paper reports novel ERP and behavioral data from an add-on pilot study of this larger clinical trial. Although the results of the Smith et al. (2016) study did not reveal robust behavioral changes (i.e., performance on neuropsychological tests, ADHD symptomatology), as only one measure of working memory suggested a potential training effect, it is still possible that observable changes may be discerned via ERP measures since changes in the brain may come before changes in behavior and testing this possibility is important to more thoroughly evaluate this novel intervention. The goals of this study were two-fold. First, we aimed to evaluate the impact of IBBS on ERPs of attentional control (P3 & N2) in young children with ADHD considering IBBS was designed to specifically target these executive functions and treatment components of IBBS (i.e., CWMT & physical exercise) have been found to improve these ERP measures (Johnstone et al., 2010; Ludyga et al., 2017; Pontifex et al., 2013). Specifically, we predicted that children randomized to IBBS would show increases in the P3 and N2 amplitudes for Go and No-Go trials following treatment as compared to TAU. We also evaluated whether the No-Go N2 effect (No-Go N2 > Go N2) emerged following treatment with IBBS since this outcome has been found in the extant literature for children with ADHD treated with stimulant medication (Broyd et al., 2005). On an exploratory basis, changes in P3 and N2 latencies were examined, as discrepant findings have been reported within and across medication studies, especially for the N2 (Liu et al., 2017; Ozdag et al., 2004; Sunohara et al., 1999). Second, we aimed to test group differences between children with and without ADHD on ERP and behavioral performance measures (No-Go accuracy, Go reaction time, Go reaction time variability) obtained during a Go/No-Go task. It was of interest to determine if the same brain abnormalities found when comparing ADHD children to HC children would also be improved by IBBS.
2. Methods
2.1. Participants
This study included 2 groups of participants; children with ADHD who participated in the large-scale clinical trial of IBBS (Title: Integrated Brain, Body, and Social (IBBS) intervention for Attention-Deficit/Hyperactivity Disorder; http://clinicaltrials.gov/ct2/show/NCT01542528) and a matched sample of typically developing children without any psychological disorders. The first group of participants recruited from the IBBS efficacy study met the following inclusion criteria: 1) age between 5 and 9 years, 2) confirmed DSM-IV-TR diagnosis of ADHD or subthreshold diagnosis of ADHD (one symptom below diagnostic criteria), 3) IQ of 80 or above, and 4) stable dose of ADHD medication for one month (if applicable). Exclusion criteria included: 1) history of a neurological disorder, concussion, or head injury, 2) severe or impairing comorbid psychological diagnosis requiring immediate therapeutic attention (e.g., psychosis, acute behavior problems, bipolar disorder), 3) psychotropic medication other than that prescribed for ADHD, and 4) motor or visual impairment that would prevent participation in the IBBS intervention.
A total of thirty-seven participants agreed to participate in the add-on EEG study, representing 40% of the original IBBS clinical trial sample. Prior to randomization, one participant dropped out of the IBBS study. Of the remaining participants, 20 were randomized to IBBS and 16 were randomized to TAU. Thirty-two subjects completed endpoint assessments, resulting in a sample of 32 subjects with post-randomization EEG data. Of these, data from 3 participants were excluded from analyses due to a high level of motion resulting in an insufficient number of artifact-free No-Go trials at one of the time points (baseline, N=1 or endpoint, N=2). A final sample of 13 IBBS participants and 16 TAU participants were analyzed to evaluate the effects of the IBBS treatment on ERP measures of attentional control.
The second group of participants included thirty-seven healthy control (HC) children matched on age and gender. Following a mass mailing, the parents of these participants indicated an interest in participating in future studies and were contacted via telephone if their children matched the demographics (i.e., age, gender, residential location) of those children with ADHD who were already enrolled in the EEG study. Three of the HC participants were excluded; two for insufficient artifact-free EEG data and one for not pressing down the button hard enough so responses were consistently recorded during the Go/No-Go task. For group comparisons at baseline, the final sample comprised of 35 participants with ADHD and 34 HC participants. See Figure 1 for a CONSORT diagram depicting the flow of participants and Table 1 for demographic and clinical characteristics of the sample disaggregated by group (IBBS vs. TAU, ADHD vs. HC). Importantly, no group differences were found between these comparison groups for any of these measures, suggesting that randomization performed as expected for the IBBS and TAU groups and the ADHD and HC groups were well-matched.
Figure 1.
CONSORT Flow Diagram
Table 1.
Demographic and clinical characteristics of IBBS vs. TAU groups and ADHD vs. HC groups.
| IBBS M(SD) N=13 | TAU M(SD) N=16 | Group Differences Test statistic | ADHD M(SD) N=35 | HC M(SD) N=34 | Group Differences Test statistic | |
|---|---|---|---|---|---|---|
| Age (years) | 7.23(1.42) | 7.06(1.06) | F(1,27)=0.13, p=0.72 | 7.03(1.32) | 7.18(1.22) | F(1,67)=0.23, p=0.63 |
| Sex, N (%) | ||||||
| Male | 7(53.8) | 8(50.0) | χ2(1)=0.04, p=0.84 | 20(57.1) | 21(61.8) | χ2(1)=0.15, p=0.70 |
| Female | 6(46.2) | 8(50.0) | 15(42.9) | 13(38.2) | ||
| Race, N (%) | ||||||
| White | 5(38.5) | 7(43.8) | χ2(3)=0.36, p=0.95 | 15(42.9) | 17(50.0) | χ2(3)=1.91, p=0.59 |
| African American | 6(46.2) | 6(37.5) | 15(42.9) | 9(26.5) | ||
| Hispanic | 1(7.7) | 1(6.3) | 2(5.7) | 3(8.8) | ||
| Other | 1(7.7) | 2(12.5) | 3(8.6) | 4(11.8) | ||
| KBIT | 107.46(14.66) | 99.63(11.52) | F(1,27)=2.60, p=0.12 | 103.69(12.67) | 107.82(13.67) | F(1,67)=1.70, p=0.20 |
| Parental Education (years) | 15.46(2.29) | 14.80(2.78) | F(1,26)=0.46, p=0.50 | 15.24(2.55) | 15.31(2.53) | F(1,64)=0.02, p=0.90 |
| ADHD Subtype, N (%) | ||||||
| Inattentive | 5(38.5) | 5(31.3) | χ2(3)=0.51, p=0.92 | --- | --- | --- |
| Hyperactive-Impulsive | 2(15.4) | 2(12.5) | --- | --- | --- | |
| Combined | 4(30.8) | 7(43.8) | --- | --- | --- | |
| Subthreshold for ADHD | 2(15.4) | 2(12.5) | --- | --- | --- | |
| ADHD Medication, N (%) | ||||||
| Stimulants | 2(15.4) | 3(18.8) | p=0.60a | --- | --- | --- |
| Non-stimulants | 1(7.7) | 1(6.3) | p=0.70a | --- | --- | --- |
| Comorbidity, N (%) | ||||||
| Anxiety Disorder | 1(7.7) | 5(31.3) | p=0.14a | --- | --- | --- |
| Oppositional Defiant Disorder | 2(15.4) | 4(25.0) | p=0.44a | --- | --- | --- |
| Autism Spectrum Disorder | 1(7.7) | 1(6.3) | p=0.70a | --- | --- | --- |
Abbreviations: HC = healthy control; IBBS = Integrated Brain, Body & Social Intervention; TAU = Treatment-As-Usual; M (SD) = mean (standard deviation); N = number of participants; KBIT = Kaufman Brief Intelligence Test.
Fisher’s Exact Test
2.2. Procedure
The present study was approved by the Yale University Human Investigation Committee and by the school district where the IBBS intervention was implemented. Informed written consent and assent was obtained from the parents and children participating in this study prior to the commencement of any study procedures. Participants were invited to take part in the add-on EEG study at their baseline assessment for the larger IBBS study. During this baseline assessment, eligibility status was determined primarily by means of a medical history (past & current medical conditions, treatments) and a semi-structured clinical interview (Kiddie Schedule for Affective Disorders and Schizophrenia–Present and Lifetime Version [K-SADS-PL]; Kaufman et al., 1997) administered by a master or doctoral-level clinician. A neurocognitive assessment battery, a measure of general intellectual ability (Kaufman Brief Intelligence Test, Second Edition [KBIT-2]; Kaufman and Kaufman, 2004), and parent-rated and clinician-rated ADHD symptom checklists (Swanson, Nolan, and Pelham Rating Scale [SNAP]; Swanson, 1992) were also completed at this assessment visit. Randomization to IBBS or TAU (stratified by medication status) occurred after the baseline assessment and within the context of the larger IBBS clinical trial. For those randomized to IBBS, the intervention lasted for 15 weeks, 3 days per week for 2 hours in an after-school setting and was implemented by school personnel (i.e., teachers, school counselors) during the first half of the school year. They also were expected to maintain their ADHD treatment regimen (e.g., medication, psychotherapy, school accommodations) that was initiated prior to their enrollment into the study. The training of school personnel involved two didactic workshops each lasting 3 hours followed by the research team modeling the treatment components until school personnel were able to independently implement the program. This training was supplemented by weekly meetings with the research team to answer any questions or address any difficulties with program implementation that arose during the study period.
The TAU group were instructed to continue with the same interventions already in place for their ADHD, but refrain from modifying or adding anything new to their treatment regimen for the duration of the study. Their compliance to this request was verified by a re-evaluation of their treatment regimen after the 15 week wait-list period. The TAU group had the option of participating in the IBBS intervention during the second half of the school year after their endpoint assessments. The same study measures were then repeated within 4 weeks after the completion of the IBBS intervention program.
Baseline and endpoint EEG visits either occurred during the IBBS assessment visits following at least a 15-minute break or at another visit that was most convenient for families within the established time frame of the IBBS baseline and endpoint assessment visits. For those participants who were taking ADHD medication, they were asked to remain on their medication for all study visits. The visit for the add-on EEG study lasted approximately 1 hour and included three computerized tasks (i.e., Go/No-Go task, resting state task, reward-feedback task) administered during electroencephalography (EEG) recordings. Participants were closely monitored by highly trained research assistants (RAs) who oversaw stimulus presentation and data acquisition to ensure the quality of EEG data. Participants were also given stickers to promote motivation in between tasks or at pre-determined breaks programmed into the tasks, which were always administered in the same order as listed above. Participants received $40 for completing EEG study procedures at each visit.
The HC participants were assessed for eligibility by trained master or doctoral-level clinicians after expressing an interest in the EEG study after initial phone contact. The absence of a neurological condition, past head trauma, and past or current psychiatric diagnosis was confirmed by a clinical interview and parent-rated forms assessing ADHD and related symptomatology during a one-time study visit. All HC participants were medication naïve. The EEG study procedures completed by the HCs were identical to the procedures completed by the IBBS study participants. They also received $40 compensation for their time and effort.
2.3. IBBS Intervention
As mentioned previously, the IBBS intervention is comprised of three treatment components. The brain component of IBBS includes three to five child-friendly computer games that at their most basic level resemble common neuropsychological tests (e.g., Continuous Performance Task, Wisconsin Card Sorting Task). Each game consists of hundreds of levels with each level building upon itself, thereby placing greater cognitive demand on the participant. The body component is a set of physical exercises designed to train the same cognitive abilities in the context of whole body activity and social activation as the brain component. The physical exercises (e.g., balance training, relay races, ball skills, aerobic dance, team sports) progress gradually from simple to more complicated movements, thereby training additional cognitive abilities as they increase in complexity. Lastly, the social component (Good Behavior Game; GBG) is the only component of treatment not specifically designed for IBBS. When playing the GBG, children worked as a team to follow the rules of the program (e.g., “We will try our best”, “We will follow instructions”) and were rewarded for their efforts (e.g., trip to the prize box, game of “follow the leader”). The overall aim of the GBG was to reduce disruptive behaviors that might interfere with the other components of IBBS and promote generalization to other settings (school, home, community).
The IBBS intervention program lasted 15 weeks and was implemented in an after-school setting 3 days a week for 2 hours. The social component was simultaneously carried out while the participants completed the other treatment components (45 minutes of computer games/brain component and 45 minutes of physical exercises/body component). School personnel were always present during the brain and body training in order to implement the social component of treatment, answer any questions the children might have, or suggest alternative strategies to improve their performance. A more detailed description of the IBBS intervention and training of school personnel is presented in our previous report focusing on the treatment effects of IBBS versus TAU on neuropsychological tests and ADHD symptoms (Smith et al., 2016).
2.4. Measures
2.4.1. Go/No-Go Task
This 15-minute Go/No-Go task has been well-piloted in neuroimaging and electrophysiological studies with children in the age range and diagnostic classification of the present study. In this version of the task, participants were presented with every day, neutral objects (e.g., furniture, clothing) enclosed in red or green frames. Participants were directed to press a button if the frame was green (Go condition) and withhold this response if the frame was red (No-Go condition). Stimuli had a minimum presentation time of 800ms and a maximum presentation time of 1150ms with an inter-trial interval ranging from 500–1500ms. Go and No-Go stimuli were presented pseudo-randomly with at least 3 Go trials proceeding a No-Go trial to build up a strong pre-potent response. Following a short practice, two blocks of 150 trials were completed consisting of 240 Go trials (75%) intermixed with 60 No-Go trials (25%). An earned points display was presented after every 25 trials and was calculated based on participant performance when correctly responding to Go (worth 1 point) and No-Go (worth 2 points) stimuli. The task resumed by pressing the spacebar on a keyboard controlled by the RA overseeing administration. E-prime 2.0 software (Psychology Software Tools, Inc.) was used to control stimulus presentation and record the accuracy and reaction time of responses. The behavioral measures of interest for this task were No-Go accuracy, Go reaction time, and Go reaction time variability.
2.4.2. EEG data acquisition and preprocessing
EEG channels were recorded during the Go/No-Go task using a high density array of 128 Ag/AgCl electrodes arranged into a net (Geodesic Sensor Net, EGI Inc., Eugene, Oregon) with a sampling rate of 250Hz by means of high impedance amplifiers (0.01 Hz high-pass, 100 Hz low-pass). Impedances were kept at or below 40kohms and all electrodes were referenced to Cz during recording. Netstation 4.4 software package (EGI, Inc.) was used to record and preprocess all EEG data.
Following data acquisition, data were filtered with a 30-Hz low-pass filter and segmented to epochs of 100ms before and 1100ms after stimulus onset. Segments with extreme voltage fluctuations defined as exceeding a threshold of 200 μV were marked as bad segments. Channels with greater than 40% bad segments were marked as bad channels. Bad channels and segments were replaced by spline interpolation. Eye blinks and eye movements were detected when vertical or horizontal eye channels, respectively, exceeded a threshold of 150 μV. Ocular artifact removal (OAR) was implemented to correct eye movements/blinks for all participants (Gratton, Coles & Donchin, 1983). Trials were then re-referenced from Cz to an average reference, baseline corrected to a 100ms pre-stimulus interval, and averaged within each condition for each participant. Trials with more than 10 bad channels were rejected. A minimum of 10 usable ERP No-Go trials were required for analyses, which is a threshold that has been used in other ERP studies evaluating the impact of CWMT interventions (e.g., Liu et al., 2017). An average of 29.47 segments (SD = 10.99, Range = 12–53) were retained for the ADHD group and an average of 29.88 segments (SD = 10.88, Range = 12–55) were retained for the HC group.
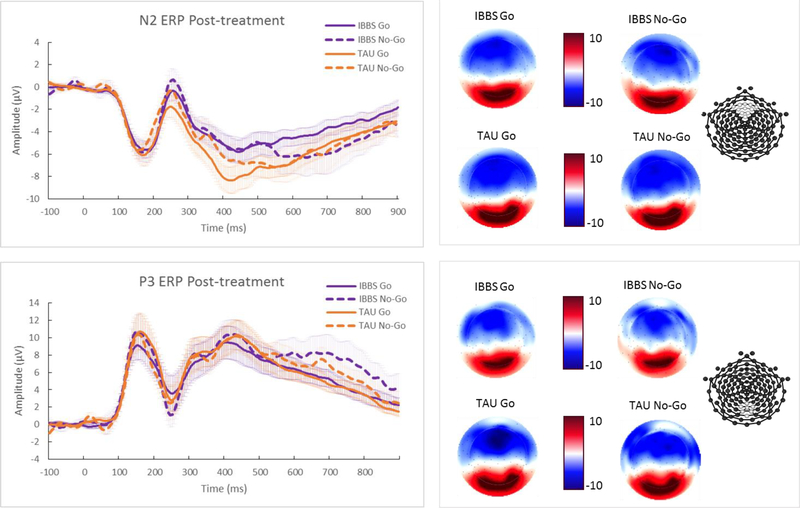
Automatic detection identified peak amplitude and latency for the stimulus-locked component of N2 at frontocentral sites (average signal recorded at Fz and surrounding 10 electrodes; Figure 2) between 100 and 300ms after stimulus onset for correct Go and No-Go trials. Average amplitude and peak latency was used for the P3 at posterior parietal sites (average signal recorded at Pz and surrounding 6 electrodes; Figure 2) between 300 and 900ms after the stimulus for correct Go and No-Go trials. Decisions of where and when to look for these ERP components were based on prior research with ADHD children and their typically developing peers using similar Go/No-Go tasks during EEG recordings (e.g., Groom et al., 2010; Wiersema et al., 2006) including the larger time window for the P3, which best captured this ERP component for the full sample.
Figure 2.
ERP grand average waveforms for IBBS and TAU groups post-treatment. Standard errors for mean ERP curves are indicated using shaded colors. Scalp topography of mean ERP values during established time window for N2 and P3 is presented to right of waveforms. Electrode plot indicates the location of the electrodes for these ERP time courses. Graphed waveforms reveal the Go P3 latency is significantly earlier for the IBBS group as compared to the TAU group.
2.5. Statistical Analyses
Prior to conducting analyses, the assumption of normality was evaluated for the ERP and behavioral measures by graphically reviewing their distributions via Boxplots and calculating skewness and kurtosis values. Two measures at time 1 and three measures at time 2 violated the assumption of normality and were also found to have outliers. Once these outliers were handled by means of winsorization (Wilcox, 2012), the values for skewness and kurtosis fell within acceptable limits.
A series of ANCOVAs were used to test the difference between IBBS versus TAU at time 2 for all ERP measures of attentional control. For each ERP measure, the model included its measurement at time 2 (post-treatment) as the dependent variable, group (IBBS vs. TAU) as the independent variable, and its measurement at time 1 (pre-treatment) as a covariate. The ANCOVA method was selected, as this approach has been shown to have more power than Repeated Measures ANOVAs for the analysis of small sample randomized controlled trials with two assessment time points (Rausch et al., 2003; van Breukelen, 2013). Further, ANCOVA methods have been used for similar study designs investigating the treatment effects of CWMT interventions for ADHD (e.g., Solanto et al., 2010; van Dongen-Boomsma et al., 2014). Separate ANCOVAs were run for Go and No-Go trials for each outcome measure. The same ANCOVA models were then conducted to test changes in the Go/No-Go behavior measures following treatment. Potential covariates were identified by correlating demographic (e.g., SES, IQ) and clinical characteristic variables (e.g., medication status, ADHD subtype, comorbidities) with study outcome variables. Based on the results of these correlations, IQ, medication status, and ADHD subtype were entered as covariates in the ANCOVA models; however, their effects were not significant so our results are presented without their inclusion.
Group comparisons between the ADHD and HC groups on demographic characteristics, ERP measures, and behavior measures at time 1 were made using one-way ANOVAs for continuous variables and chi-square tests for categorical variables. Cohen’s d (i.e., the difference between the change scores of each group divided by the pooled standard deviations at baseline) was calculated as a measure of effect size (Morris, 2008). Given this was a pilot study with a relatively small sample, the results of this study are reported without controlling for multiple comparisons.
3. Results
3.1. IBBS treatment effects on study measures
There were no significant treatment effects found for the N2 and P3 amplitudes for Go or No-Go trials. However, a significant treatment group difference was found for Go P3 latency, F(1,26)=5.35, p=0.03, d=1.12, such that the IBBS group had a significantly earlier P3 response (M=336.09, SD=32.42) relative to the TAU group (M=437.04, SD=155.95) following treatment. The N2 and P3 difference waves, serving as direct tests of the No-Go N2 effect (No-Go N2 > Go N2) and No-Go P3 effect (No-Go P3 > Go P3), also did not reveal any significant group differences post-treatment (N2 difference wave: F(1,26)=0.73, p=0.40, d=0.54; P3 difference wave: F(1,26)=1.45, p=0.24, d=0.17). On an exploratory basis, the same analytic approach used by Broyd and colleagues (2005) to evaluate the No-Go N2 effect and No-Go P3 effect was employed where the Go and No-Go amplitudes for each ERP at time 2 were compared for the IBBS and TAU groups separately. A comparison of Go and No-Go N2 amplitudes at time 2 did not reveal any significant differences for either group (IBBS group: t(12)=1.61, p=0.27, d=0.21, TAU group: t(15)=0.19, p=0.85, d=0.03); however, the No-Go P3 amplitude was found to be significantly larger than the Go P3 amplitude for the IBBS group following treatment, t(12)=2.10, p=0.05, d=0.41, but no significant difference between Go and No-Go P3 amplitudes was found for the TAU group, t(15)=0.65, p=0.53, d=0.16. There were no significant treatment effects for any of the behavioral measures (i.e., Go accuracy, No-Go accuracy, Go RT, and Go RT variability), F(1,26)=0.09–1.33, p=0.26–0.77, d=−0.02–0.49. Detailed descriptive and ANCOVA test statistics are presented in Table 2. The ERP waveforms for the IBBS and TAU groups after treatment are presented in Figure 2 whereas the ERP waveforms for these groups before treatment are shown in Figure S1.
Table 2.
Test of group wise (IBBS vs. TAU) treatment differences for ERP and behavioral measures.
| Measures | IBBS N | IBBS-baseline M(SD) | IBBS-endpoint M(SD) | TAU N | TAU-baseline M(SD) | TAU-endpoint M(SD) | Test statistic | Effect sizea | p-value |
|---|---|---|---|---|---|---|---|---|---|
| ERP Measures | |||||||||
| Go N2 Amplitude | 13 | −8.61(3.24) | −7.44(2.29) | 16 | −9.30(3.83) | −8.52(3.79) | F(1,26)=0.54 | −0.108 | 0.468 |
| No-Go N2 Amplitude | 13 | −7.40(2.86) | −8.10(3.32) | 16 | −8.42(3.89) | −8.70(5.92) | F(1,26)=0.06 | 0.120 | 0.814 |
| N2 Difference Wave | 13 | 1.21(2.80) | −0.66(2.05) | 16 | 0.87(1.72) | 0.24(3.44) | F(1,26)=0.73 | 0.542 | 0.402 |
| Go N2 Latency | 13 | 223.38(75.40) | 194.69(72.70) | 16 | 240.32(63.88) | 236.55(67.02) | F(1,26)=2.23 | 0.499 | 0.147 |
| No-Go N2 Latency | 13 | 201.31(51.84) | 185.20(64.44) | 16 | 212.50(59.07) | 187.98(75.80) | F(1,26)=0.02 | −0.149 | 0.883 |
| Go P3 Amplitude | 13 | 7.01(5.79) | 3.65(2.90) | 16 | 5.85(4.67) | 4.24(3.62) | F(1,26)=1.24 | −0.333 | 0.276 |
| No-Go P3 Amplitude | 13 | 8.24(6.64) | 5.84(5.41) | 16 | 6.25(6.24) | 4.91(4.82) | F(1,26)=0.06 | −0.163 | 0.812 |
| P3 Difference Wave | 13 | 1.22(4.42) | 2.19(3.77) | 16 | 0.40(3.61) | 0.67(4.12) | F(1,26)=1.45 | 0.174 | 0.240 |
| Go P3 Latency | 13 | 374.59(120.77) | 336.09(32.42) | 16 | 366.68(71.10) | 437.04(155.95) | F(1,26)=5.35 | 1.118 | 0.029 |
| No-Go P3 Latency | 13 | 428.35(117.76) | 409.05(76.50) | 16 | 454.25(155.64) | 434.07(112.23) | F(1,26)=0.37 | 0.006 | 0.548 |
| Behavioral Measures | |||||||||
| Go Accuracy | 13 | 0.90(0.09) | 0.91(0.08) | 16 | 0.90(0.06) | 0.88(0.10) | F(1,26)=1.33 | 0.397 | 0.260 |
| No-Go Accuracy | 13 | 0.79(0.14) | 0.78(0.17) | 16 | 0.82(0.08) | 0.77(0.11) | F(1,26)=0.34 | 0.357 | 0.564 |
| Go RT | 13 | 510.54(59.22) | 458.62(66.38) | 16 | 524.37(76.75) | 470.91(58.05) | F(1,26)=0.09 | −0.022 | 0.766 |
| Go RT Variability | 13 | 138.69(27.45) | 123.30(17.92) | 16 | 132.78(30.35) | 131.78(28.56) | F(1,26)=1.24 | 0.490 | 0.275 |
Abbreviations: IBBS = Integrated Brain, Body, and Social Intervention; TAU = Treatment-As-Usual; N = number of participants; M = mean; SD = standard deviation; ERP = event-related potentials; RT = reaction time
Cohen’s d
3.2. Group comparisons of ADHD versus HC groups
As presented in Table 3, no significant group differences (ADHD vs. HC) were found for the N2 and P3 amplitudes or latencies for Go and No-Go trials. However, the N2 difference wave was significantly larger for the ADHD group as compared to the HC group, F(1, 67)=6.82, p=0.01, d=0.63. To further examine this finding, the Go and No-Go N2 amplitudes at time 1 were compared for the ADHD and HC groups separately. For the ADHD group, the Go N2 amplitude was significantly larger than the No-Go N2 amplitude, t(34)=−2.63, p=0.01, d=−0.28, whereas there was no significant difference between Go and No-Go N2 amplitudes for the HC group, t(33)=0.98, p=0.34, d=0.09. Thus, neither the ADHD group nor the HC group evidenced a No-Go N2 effect (No-Go N2 > Go N2), but this effect was in the opposite direction for the ADHD group (Go N2 > No-Go N2). Although the P3 difference wave was not significantly different across groups (ADHD vs. HC), F(1, 67)=2.69, p=0.11, d=0.40, the No-Go P3 amplitude was found to be significantly larger than the Go P3 amplitude for the HC group, t(33)=3.41, p=0.002, d=0.58; however, no significant difference was revealed for the ADHD group, t(34)=1.23, p=0.23, d=0.14. Figure S2 provides a graphical depiction of ERP waveforms for the ADHD and HC groups at baseline.
Table 3.
Group differences between ADHD and HC groups for ERP and behavioral measures.
| Measures | ADHD M(SD) N = 35 | HCM(SD) N = 34 | Test statistic | Effect sizea | p-value |
|---|---|---|---|---|---|
| ERP Measures | |||||
| Go N2 Amplitude | −9.18(3.35) | −8.91(3.29) | F(1,67)=0.12 | −0.271 | 0.744 |
| No-Go N2 Amplitude | −8.24(3.36) | −9.21(3.43) | F(1,67)=1.44 | 0.286 | 0.235 |
| N2 Difference Wave | 0.94(2.11) | −0.31(1.83) | F(1,67)=6.82 | 0.634 | 0.011 |
| Go N2 Latency | 231.02(68.74) | 208.75(74.73) | F(1,67)=1.66 | 0.310 | 0.202 |
| No-Go N2 Latency | 205.88(58.03) | 193.07(68.68) | F(1,67)=0.70 | 0.202 | 0.405 |
| Go P3 Amplitude | 6.00(5.01) | 4.11(2.55) | F(1,67)=3.85 | −0.473 | 0.055 |
| No-Go P3 Amplitude | 6.78(5.95) | 6.41(4.57) | F(1,67)=0.08 | −0.070 | 0.773 |
| P3 Difference Wave | 0.78(3.76) | 2.30(3.92) | F(1,67)=2.69 | 0.396 | 0.106 |
| Go P3 Latency | 383.36(115.22) | 355.53(52.38) | F(1,67)=1.65 | 0.309 | 0.203 |
| No-Go P3 Latency | 438.38(129.31) | 392.07(95.75) | F(1,67)=2.85 | 0.406 | 0.096 |
| Behavioral Measures | |||||
| Go Accuracy | 0.90(0.08) | 0.92(0.08) | F(1,67)=0.44 | 0.250 | 0.511 |
| No-Go Accuracy | 0.79(0.11) | 0.76(0.12) | F(1,67)=1.55 | −0.261 | 0.217 |
| Go RT | 512.84(70.06) | 462.16(55.41) | F(1,67)=11.07 | 0.801 | 0.001 |
| Go RT Variability | 133.88(28.58) | 124.56(21.96) | F(1,67)=2.30 | 0.381 | 0.134 |
Abbreviations: M = mean; SD = standard deviation; N = number of participants; ERP = event-related potentials; RT = reaction time
Cohen’s d
With respect to the behavioral measures, a significant group difference was found for Go reaction time (RT), F(1, 67)=11.07, p=0.001, d=0.80, where the ADHD group (M=512.84, SD=70.06) had a slower RT than the HC group (M=462.16, SD=55.41). There were no significant group differences for the remaining Go/No-Go behavioral measures (Go accuracy, No-Go accuracy, RT variability).
4. Discussion
The primary goal of this study was to examine whether changes in ERP measures of attentional control (P3 and N2) were achieved following treatment with a novel, multi-faceted cognitive training intervention (IBBS) designed to target underlying EF deficits associated with ADHD. A significant change in ERP measures was expected given that the intervention was carried out via two training modalities (brain and body) and the IBBS exercises made use of several higher-order cognitive processes (e.g., sustained attention, response inhibition) that are theorized to comprise EFs and have been found to be less developed in children with ADHD (Crippa et al., 2015; Willcutt et al., 2005). It was also of interest to evaluate whether our pattern of results from a sample of young children (aged 5 to 9 years) approximated the ADHD and HC significant group differences found for ERP and behavioral measures in prior studies with older participants (Albrecht et al., 2008; Brandeis et al., 2002; Wiersema et al., 2006; Wilcutt et al., 2005). Such an objective is worthwhile if the same brain abnormalities identified when comparing our sample of ADHD participants to HC participants are normalized following treatment with IBBS, as this outcome would offer compelling evidence that IBBS is able to affect underlying neural correlates of EF deficits in ADHD.
4.1. IBBS impact on ERP measures
Most notably, we found a significant treatment group difference for Go P3 latency such that the P3 response for Go trials was earlier for the IBBS group as compared to the TAU group (336.09 vs. 437.04). Inspection of the means revealed that the Go P3 latency slightly decreased (became earlier) for the IBBS group (374.59 to 336.09) whereas the Go P3 latency sharply increased (became later) for the TAU group (366.68 to 437.04). Assuming shorter latency represents more efficient processing, such a pattern of results suggests that IBBS may prevent the worsening of discrimination processing abilities, which requires both attentional and effortful control (Barry et al., 2003; Johnstone et al., 2001), in children with ADHD. Indeed, studies have suggested that EF deficits are associated with the continuity of ADHD over time (e.g., Brocki et al., 2007) and prevention efforts may need to target early phenotypes of ADHD to alter the developmental trajectory of this disorder. Our finding of the effect of IBBS versus TAU on the change in Go P3 latency offers a potential biomarker of attentional control that may become less efficient across development without interventions for ADHD. However, given the small sample size and large number of analyses necessitated by the ten ERP measures generated by the Go/No-Go task, this result requires replication in an independent sample.
The N2 and P3 difference waves were used to evaluate if a No-Go N2 effect (No-Go N2 > Go N2) or No-Go P3 effect (No-Go P3 > Go P3) would emerge following treatment with IBBS, as their absence is believed to reflect an abnormality in attentional control (Barry et al., 2003; Holcomb et al., 1986). Although a significant group difference was not found for the N2 and P3 difference waves, exploratory analyses revealed that the No-Go P3 amplitude was significantly larger than the Go P3 amplitude for the IBBS group, but not the TAU group post-treatment. This finding is of importance since the ADHD group did not show a significant difference between the No-Go P3 amplitude and Go P3 amplitude at baseline whereas the HC group did in the expected direction (No-Go P3 > Go P3). It is quite possible that our study was underpowered to detect a significant treatment group difference for the P3 difference wave, but the results of our exploratory analyses suggest that re-examining the impact of IBBS on the No-Go P3 effect (No-Go P3 > Go P3) in a larger sample is warranted.
Contrary to our predictions and the results of intervention studies employing medication and non-pharmacological treatments similar to IBBS (Groom et al., 2010; Ozdag et al., 2004; Johnstone et al., 2010; Ludyga et al., 2017), significant increases in P3 and N2 amplitudes were not found for the IBBS group as compared to the TAU group post-treatment. These results are consistent with a study that examined P3 and N2 amplitude changes following CWMT in a sample of adults (Liu et al., 2017). In fact, the only significant group difference found by Liu and colleagues (2017) for these ERP measures was an increase in the Go N2 amplitude for the waitlist control group relative to the two active treatment conditions (standard length & shortened length CWMT).
4.2. ADHD and HC group differences for ERP measures
When comparing children with ADHD to HC children on ERP measures, a significant group effect for the N2 difference wave (No-Go minus Go) was found, indicating the magnitude of the difference between the Go N2 amplitude and No-Go N2 amplitude for the ADHD group was significantly larger than the HC group. To further examine these results, the Go N2 amplitude was found to be significantly larger than the No-Go N2 amplitude for the ADHD group, which is consistent with the finding of Broyd and colleagues (2005) and suggestive of a potential abnormality. Interestingly, our study found no significant differences between the Go N2 amplitude and No-Go N2 amplitude for the HC group implying less developed attentional control at this stage of development since a No-Go N2 effect (No-Go N2 > Go N2) is usually found in older samples of typically developing children (8 to 11 years; Broyd et al., 2005). We also did not replicate findings of attenuated P3 and N2 amplitudes for children with ADHD as compared to HC children, which may be attributable to the young age of our sample as well.
4.3. Group differences for behavioral measures
As expected, our findings on behavioral measures were consistent with previous research (Wilcutt et al., 2005), as the ADHD group showed significantly slower response times as compared to the HC group. However, we did not find a treatment effect in favor of IBBS over TAU on any of the behavioral measures, which mirrors the findings of the larger RCT of IBBS since a full battery of neuropsychological tests did not find significant differences across treatment groups that survived corrections for multiple comparisons (Smith et al., 2016). It is relevant to note that behavioral measures in the present study were limited to the Go/No-Go task done in the context of the EEG recordings, with the relative absence of other distractions and in the presence of an RA providing individual attention. Since behavioral manifestations of ADHD are highly sensitive to context, neuropsychological tests that are administered in settings approximating environments in which children with ADHD struggle (e.g., more cognitive demands or extraneous stimuli) may be more sensitive to ADHD interventions such as IBBS.
4.4. Limitations and Future Directions
Considering IBBS is comprised of intervention components (i.e., physical exercise, Good Behavior Game) known to improve ADHD symptomatology and associated EF deficits (Blair and Diamond, 2008; Grassmann et al., 2014; Leflot et al., 2010; Spilt et al., 2016; Verret et al., 2012), it is important to discuss why additional changes on ERP measures were not found. First, it is possible that the neurocognitive component of IBBS did not make use of the same neural pathway engaged by the Go/No-Go paradigm or that engagement without real-world application is not enough to evidence significant change. Second, the body component focused on skill acquisition rather than periods of aerobic physical exercise and perhaps there is a certain threshold of energy that must be exerted in order to produce treatment effects (Ludyga et al., 2017; Ng et al., 2017). The social component of IBBS (Good Behavior Game; GBG) was used to limit off-task and disruptive behaviors during the brain and body components of treatment. However, the GBG is typically implemented for the entire school year with participants’ classmates. These two implementation procedures for the GBG were not adopted when IBBS was put into practice as an after-school program. Finally, the timing and dosage of the intervention may have been sub-optimal to result in improvements across multiple outcome measures in favor of IBBS.
The main limitation of this study is its small sample size, thus increasing the likelihood of a Type II error, which influenced our decision to not correct for multiple comparisons. As a result, it is possible that some of our findings may be due to chance and underscores the importance of replication in a larger sample. However, pilot studies are important to inform the development of larger investigations and this study may be helpful in the planning of future studies of non-pharmacological interventions for children with ADHD and collecting EEG data as outcome measures in these trials. The second limitation of the study is the multicomponent nature of the IBBS intervention, which makes it impossible to determine which element may have been sub-optimal to improve performance on neuropsychological tasks of sustained attention and their underlying neural processes captured by N2 and P3 ERP measures. This limitation may be addressed by studying the effects of more homogenous behavioral and cognitive interventions on specific executive functions and their neural underpinnings or adding control remediations (e.g., cognitive training targeting one EF, one type of physical exercise) to the research design. The third limitation of this study is a lack of a control condition where participants receive some sort of active intervention (e.g., non-adaptive version of CWMT). Thus, we were unable to control for non-specific effects of the intervention (e.g., expectation of improvement, rapport/alliance between participant and treatment provider). Future studies are encouraged to employ active control conditions as a way to more rigorously evaluate IBBS and similar types of ADHD interventions. Finally, although our criterion for the number of usable ERP No-Go trials required for analysis was more lenient than recommendations made in the extant adult literature (e.g., Duncan, 2009), it was dictated by the practical demands of conducting a study with children as young as 5 years of age and is comparable to other ERP studies with pediatric populations (e.g., Rahman et al., 2017).
4.5. Conclusion
Although this pilot study did not reveal robust treatment effects, it suggests that IBBS may prevent the worsening of attentional control systems in the brain, as evidenced by the significant treatment group difference for Go P3 latency. Larger studies would allow for a better examination of change in ERP measures following IBBS treatment and if changes in ERP measures translate into improved behavioral performance.
Supplementary Material
Highlights.
Brain attentional control networks may show changes following treatment with IBBS
Go P3 latency and No-Go P3 effect should be used to evaluate ADHD treatment response
Impact of IBBS on ERP measures requires further study in a larger sample
Acknowledgments
Funding for this project was provided by the Director’s Office at the National Institutes of Health (R01HD070821; PIs: Leckman & Wexler) and the Brain and Behavior Research Foundation (NARSAD Grant #22288; PI: Smith). The funding sources had no involvement in the study design, the collection and analysis of the data, or the writing of the manuscript.
Abbreviations
- ERP
event-related potentials
- TAU
treatment-as-usual
- EEG
electroencephalography
- HC
healthy controls
- ADHD
Attention-Deficit/Hyperactivity Disorder
- EF
executive function
- RA
research assistant
Footnotes
Conflict of Interest: Dr. Bruce Wexler is chief scientist and holds equity in C8 Sciences, which developed and sells the brain training program evaluated by the research described in this paper. Dr. James Leckman receives royalties from John Wiley and Sons, McGraw Hill, and Oxford University Press; is on the Advisory Boards for Brain and Behavior Research Foundation and How I Decide; and has served as a consultant for Tasly Pharmaceuticals, Inc. Dr. Sukhodolsky receives royalties from Guilford Press. All remaining authors have declared no competing or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramovitch A, Goldzweig G, Schweiger A, 2013. Correlates of physical activity with intrusive thoughts, worry and impulsivity in adults with attention deficit/hyperactivity disorder: A cross-sectional pilot study. Isr J Psychiatry Relat Sci. 50(1), 47–54. [PubMed] [Google Scholar]
- Albrecht B, Brandeis D, Uebel H, Heinrich H, Mueller UC, Hasselhorn M et al. , 2008. Action monitoring in boys with attention-deficit/hyperactivity disorder, their nonaffected siblings, and normal control subjects: evidence for an endophenotype. Biol Psychiatry 64(7), 615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry RJ, Johnstone SJ, Clarke AR, 2003. A review of electrophysiology in attention-deficit/hyperactivity disorder: II. Event-related potentials. Clin Neurophysiol 114(2), 184–198. [DOI] [PubMed] [Google Scholar]
- Bigorra A, Garolera M, Guijarro S, Hervás A, 2016. Long-term far-transfer effects of working memory training in children with ADHD: a randomized controlled trial. Eur Child Adolesc Psychiatry 25(8), 853–867. [DOI] [PubMed] [Google Scholar]
- Blair C, Diamond A, 2008. Biological processes in prevention and intervention: The promotion of self-regulation as a means of preventing school failure. Dev Psychopathol, 20(3), 899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandeis D, Banaschewski T, Baving L, Georgiewa P, Blanz B, Schmidt MH et al. , 2002. Multicenter P300 brain mapping of impaired attention to cues in hyperkinetic children. J Am Acad Child Adolesc Psychiatry 41(8), 990–998. [DOI] [PubMed] [Google Scholar]
- Brocki KC, Nyberg L, Thorell LB, & Bohlin G (2007). Early concurrent and longitudinal symptoms of ADHD and ODD: Relations to different types of inhibitory control and working memory. J. Child Psychol. Psychiatry, 48, 1033–1041. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, Johnstone SJ, Barry RJ, Clarke AR, McCarthy R, Selikowitz M et al. , 2005. The effect of methylphenidate on response inhibition and the event-related potential of children with attention deficit/hyperactivity disorder. Int J Psychophysiol 58(1), 47–58. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R, 2006. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn. Sci. 10(3), 117–123. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, & Tannock R (2002). Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci 3(8), 617. [DOI] [PubMed] [Google Scholar]
- Chacko A, Bedard AC, Marks DJ, Feirsen N, Uderman JZ, Chimiklis A et al. , 2014. A randomized clinical trial of Cogmed Working Memory Training in school-age children with ADHD: a replication in a diverse sample using a control condition. J Child Psychol Psychiatry 55(3), 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkasova M, Sulla EM, Dalena KL, Pondé MP, Hechtman L, 2013. Developmental course of attention deficit hyperactivity disorder and its predictors. J Can Acad Adolesc Psychiatry 22(1), 47–54. [PMC free article] [PubMed] [Google Scholar]
- Crippa A, Marzocchi GM, Piroddi C, Besana D, Giribone S, Vio C et al. , 2015. An integrated model of executive functioning is helpful for understanding ADHD and associated disorders. J Atten Disord 19(6), 455–467. [DOI] [PubMed] [Google Scholar]
- Dalsgaard S, Østergaard SD, Leckman JF, Mortensen PB, Pedersen MG, 2015. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: A nationwide cohort study. Lancet 385(9983), 2190–2196. [DOI] [PubMed] [Google Scholar]
- Donkers FC, Van Boxtel GJ, 2004. The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain Cogn 56(2), 165–176. [DOI] [PubMed] [Google Scholar]
- Doyle AE, Biederman J, Seidman LJ, Weber W, Faraone SV, 2000. Diagnostic efficiency of neuropsychological test scores for discriminating boys with and without attention deficit–hyperactivity disorder. J Consult Clin Psychol 68(3), 477. [DOI] [PubMed] [Google Scholar]
- Duncan CC, Barry RJ, Connolly JF, Fischer C, Michie PT, Naatanen R, et al. , 2009. Event-related potentials in clinical research: Guidelines for eliciting, recoding, and quantifying mismatch negativity, P300, and N400. Clin Neurophysiol 120(11), 1883–1908. [DOI] [PubMed] [Google Scholar]
- Fair DA, Bathula D, Nikolas MA, Nigg JT, 2012. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proc Natl Acad Sci 109(17), 6769–6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassmann V, Alves MV, Santos-Galduróz RF, Galduróz JCF, 2014. Possible cognitive benefits of acute physical exercise in children with ADHD: a systematic review. J Atten Disord 21(5), 367–371. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG & Donchin E, 1983. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol 55(4), 468–84. [DOI] [PubMed] [Google Scholar]
- Gray S, Chaban P, Martinussen R, Goldberg R, Gotlieb H, Kronitz R et al. , 2012. Effects of a computerized working memory training program on working memory, attention, and academics in adolescents with severe LD and comorbid ADHD: a randomized controlled trial. J Child Psychol Psychiatry 53(12), 1277–1284. [DOI] [PubMed] [Google Scholar]
- Green CT, Long DL, Green D, Iosif AM, Dixon JF, Miller MR et al. , 2012. Will working memory training generalize to improve off-task behavior in children with attention-deficit/hyperactivity disorder? Neurotherapeutics 9(3), 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groom MJ, Scerif G, Liddle PF, Batty MJ, Liddle EB, Roberts KL et al. , 2010. Effects of motivation and medication on electrophysiological markers of response inhibition in children with attention-deficit/hyperactivity disorder. Biol Psychiatry 67(7), 624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius P, Laasonen M, Hokkanen L, Paetau R, Niemivirta M, 2011. Impaired engagement of the ventral attentional pathway in ADHD. Neuropsychologia 49(7), 1889–1896. [DOI] [PubMed] [Google Scholar]
- Hervey AS, Epstein JN, Curry JF, 2004. Neuropsychology of adults with attention-deficit/hyperactivity disorder: a meta-analytic review. Neuropsychol 18, 485–503. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP, Carte ET, Sami N, Treuting JJ, Zupan BA, 2002. Preadolescent girls with attention-deficit/hyperactivity disorder: II. Neuropsychological performance in relation to subtypes and individual classification. J Consult Clin Psychol 70(5), 1099. [DOI] [PubMed] [Google Scholar]
- Holcomb PJ, Ackerman PT, Dykman RA, 1986. Auditory event-related potentials in attention and reading disabled boys. Int J Psychophysiol 3(4), 263–273. [DOI] [PubMed] [Google Scholar]
- Huang-Pollock CL, Maddox WT, Tam H, 2014. Rule-based and information-integration perceptual category learning in children with attention-deficit/hyperactivity disorder. Neuropsychol 28(4), 594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen TW, Bink M, Gelade K, van Mourik R, Maras A, Oosterlaan J 2016. A randomized controlled trial investigating the effects of neurofeedback, methylphenidate, and physical activity on event-related potentials in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 26(4), 244–53. [DOI] [PubMed] [Google Scholar]
- Johnstone SJ, Barry RJ, Anderson JW, 2001. Topographic distribution and developmental timecourse of auditory event-related potentials in two subtypes of attention-deficit hyperactivity disorder. Int J Psychophysiol 42(1), 73–94. [DOI] [PubMed] [Google Scholar]
- Johnstone SJ, Roodenrys S, Blackman R, Johnston E, Loveday K, Mantz S et al. , 2012. Neurocognitive training for children with and without AD/HD. Atten Defic Hyperact Disord 4(1), 11–23. [DOI] [PubMed] [Google Scholar]
- Johnstone SJ, Roodenrys S, Phillips E, Watt AJ, Mantz S, 2010. A pilot study of combined working memory and inhibition training for children with AD/HD. Atten Defic Hyperact Disord 2(1), 31–42. [DOI] [PubMed] [Google Scholar]
- Kamp CF, Sperlich B, Holmberg HC, 2014. Exercise reduces the symptoms of attention-deficit/hyperactivity disorder and improves social behaviour, motor skills, strength and neuropsychological parameters. Acta Paediatr 103(7), 709–714. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P et al. , 1997. Schedule for Affective Disorders and Schizophrenia for School-Age Children – Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36(7), 980–988. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL, 2004. Kaufman Brief Intelligence Test, fourth ed. Circle Pines, MN. [Google Scholar]
- Kelly AC, Hester R, Foxe JJ, Shpaner M, & Garavan H, 2006. Flexible cognitive control: effects of individual differences and brief practice on a complex cognitive task. Neuroimage 31(2), 866–886. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlström K, ... Westerberg H, 2005. Computerized training of working memory in children with ADHD-a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry, 44(2), 177–186. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, & Westerberg H (2002). Training of working memory in children with ADHD. J Clin Exp Neuropsychol 24(6), 781–791. [DOI] [PubMed] [Google Scholar]
- Knecht C, De Alvaro R, Martinez-Raga J, Balanza-Martinez V, 2015. Attention-deficit hyperactivity disorder (ADHD), substance use disorders, and criminality: a difficult problem with complex problems. Int J Adolesc Med Health 27(2), 163–175. [DOI] [PubMed] [Google Scholar]
- Kornell N, Metcalfe J, 2006. Study efficacy and the region of proximal learning framework. J Exp Psychol Learn Mem Cogn 32(3), 609. [DOI] [PubMed] [Google Scholar]
- Lazzaro I, Gordon E, Whitmont S, Meares R, Clarke S, 2001. The modulation of late component event related potentials by pre-stimulus EEG theta activity in ADHD. Int J Neurosci 107(3–4), 247–264. [DOI] [PubMed] [Google Scholar]
- Leflot G, van Lier PA, Onghena P, Colpin H, 2010. The role of teacher behavior management in the development of disruptive behaviors: An intervention study with the good behavior game. J Abnorm Chid Psychol 38(6), 869–882. [DOI] [PubMed] [Google Scholar]
- Liu ZX, Lishak V, Tannock R, Woltering S, 2017. Effects of working memory training on neural correlates of Go/Nogo response control in adults with ADHD: A randomized controlled trial. Neuropsychol 95, 54–72. [DOI] [PubMed] [Google Scholar]
- Loiselle DL, Stamm JS, Maitinsky S, Whipple SC, 1980. Evoked potential and behavioral signs of attentive dysfunctions in hyperactive boys. Psychophysiol 17(2), 193–201. [DOI] [PubMed] [Google Scholar]
- Lovejoy DW, Ball JD, Keats M, Stutts ML, Spain EH, Janda L, Janusz J, 1999. Neuropsychological performance of adults with attention deficit hyperactivity disorder (ADHD): Diagnostic classification estimates for measures of frontal lobe/executive functioning. J Int Neuropsychol Soc 5(3), 222–233. [DOI] [PubMed] [Google Scholar]
- Ludyga S, Brand S, Gerber M, Pühse U, 2017. Exercise as neuroenhancer in children with ADHD. Physical Activity and Educational Achievement: Insights from Exercise Neuroscience, 171. [Google Scholar]
- Mawjee K, Woltering S, Tannock R, 2015. Working memory training in post-secondary students with ADHD: A randomized controlled study. PloS One 10(9), e0137173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD, 2000. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn Psychol, 41(1), 49–100. [DOI] [PubMed] [Google Scholar]
- Morris SB (2008). Estimating Effect Sizes From Pretest-Posttest-Control Group Designs. Organ Res Methods, 11(2), 364–386. [Google Scholar]
- Ng QX, Ho CYX, Chan HW, Yong BZJ, Yeo WS, 2017. Managing childhood and adolescent attention-deficit/hyperactivity disorder (ADHD) with exercise: A systematic review. Complement Ther Med 34,123–128. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJ, 2005. Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes?. Biol Psychiatry, 57(11), 1224–1230. [DOI] [PubMed] [Google Scholar]
- Ozdag MF, Yorbik O, Ulas UH, Hamamcioglu K, Vural O, 2004. Effect of methylphenidate on auditory event related potential in boys with attention deficit hyperactivity disorder. Int J Pediatr Otorhinolaryngol 68(10), 1267–1272. [DOI] [PubMed] [Google Scholar]
- Polich J, 2007. Updating P300: An integrative theory of P3a and P3b. Clin Neurophysiol 118 (10), 2128–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontifex MB, Saliba BJ, Raine LB, Picchietti DL, Hillman CH, 2013. Exercise improves behavioral, neurocognitive, and scholastic performance in children with attention-deficit/hyperactivity disorder. J Pediatr 162(3), 543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman AA, Carroll DJ, Espy KA, Wiebe SA, 2017. Neural correlates of response inhibition in early childhood: Evidence from a Go/No-Go task. Dev Neuropsychol 42(5), 336–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapport MD, Orban SA, Kofler MJ, Friedman LM, 2013. Do programs designed to train working memory, other executive functions, and attention benefit children with ADHD? A meta-analytic review of cognitive, academic, and behavioral outcomes. Clin Psychol Rev 33(8), 1237–1252. [DOI] [PubMed] [Google Scholar]
- Rausch JR, Maxwell SE, Kelley K, 2003. Analytic methods for questions pertaining to a randomized pretest, posttest, follow-up design. J Clin Child Adolesc Psychol 32(3), 467–486. [DOI] [PubMed] [Google Scholar]
- Roberts BA, Martel MM, Nigg JT, 2017. Are there executive dysfunction subtypes within ADHD?. J Atten Disord 21(4), 284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterfield JH, Schell AM, Backs RW, Hidaka KC, 1984. A cross-sectional and longitudinal study of age effects of electrophysiological measures in hyperactive and normal children. Biol Psychiatry 19(7), 973–990. [PubMed] [Google Scholar]
- Satterfield JH, Schell AM, Nicholas T, Backs RW, 1988. Topographic Study of Auditory EventRelated Potentials in Normal Boys and Boys with Attention Deficit Disorder with Hyperactivity. Psychophysiol 25(5), 591–606. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, 2006. Dorsolateral pre-frontal and anterior cingulate abnormalities in adults with ADHD assessed with structural MRI. Biol Psychiatry, 59(8), 192S–193S. [Google Scholar]
- Seidman L, Bruder G, 2003. Neuropsychological testing and neurophysiological assessment In Tasman A, Kay J, & Lieberman J (Eds.), Psychiatry. London, UK: John Wiley and Sons. [Google Scholar]
- Seidman LJ, Toomey ROSEMARY, 1999. The clinical use of psychological and neuropsychological tests. The Harvard guide to psychiatry, 3, 40–64. [Google Scholar]
- Smith SD, Vitulano LA, Katsovich L, Li S, Moore C, Li F et al. , 2016. A randomized controlled trial of an integrated brain, body, and social intervention for children with ADHD. J Atten Disord 1, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanto MV, Marks DJ, Wasserstein J, Mitchell K, Abikoff H, Alvir JMJ et al. , 2010. Efficacy of meta-cognitive therapy for adult ADHD. Am J Psychiatry 167(8), 958–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Brandeis D, Cortese S, Daley D, Ferrin M, Holtmann M, ... Dittmann RW, 2013. Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatry 170(3), 275–289. [DOI] [PubMed] [Google Scholar]
- Spilt JL, Leflot G, Onghena P, Colpin H, 2016. Use of Praise and Reprimands as Critical Ingredients of Teacher Behavior Management: Effects on Children’s Development in the Context of a Teacher-Mediated Classroom Intervention. Prev Sci 17(6), 732–742. [DOI] [PubMed] [Google Scholar]
- Sunohara GA, Malone MA, Rovet J, Humphries T, Roberts W, Taylor MJ, 1999. Effect of methylphenidate on attention in children with attention deficit hyperactivity disorder (ADHD): ERP evidence. Neuropsychopharmacol 21(2), 218–228. [DOI] [PubMed] [Google Scholar]
- Swanson JM, 1992. School-based assessments and interventions for ADD students. Irvine, CA. [Google Scholar]
- Tye C, Asherson P, Ashwood KL, Azadi B, Bolton P, McLoughlin G, 2014. Attention and inhibition in children with ASD, ADHD and co-morbid ASD+ ADHD: an event-related potential study. Psychol Med 44(5), 1101–1116. [DOI] [PubMed] [Google Scholar]
- Vacha-Haase T, Thompson B, 2004. How to estimate and interpret various effect sizes. J Couns Psychol 51(4), 473. [Google Scholar]
- van Breukelen GJ, 2013. ANCOVA versus CHANGE from baseline in nonrandomized studies: The difference. Multivar Behav Res 48(6), 895–922. [DOI] [PubMed] [Google Scholar]
- van der Donk M, Hiemstra-Beernink AC, Tjeenk-Kalff A, van der Leij A, Lindauer R, 2015. Cognitive training for children with ADHD: a randomized controlled trial of cogmed working memory training and ‘paying attention in class’. Front Psychol 6, 1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen-Boomsma M, Vollebregt MA, Buitelaar JK, Slaats-Willemse D, 2014. Working memory training in young children with ADHD: a randomized placebo-controlled trial. J. Child Psychol. Psychiatry 55(8), 886–896. [DOI] [PubMed] [Google Scholar]
- Verret C, Guay MC, Berthiaume C, Gardiner P, Béliveau L, 2012. A physical activity program improves behavior and cognitive functions in children with ADHD: an exploratory study. J Atten Disord 16(1), 71–80. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Iseli M, Leon S, Zaggle W, Rush C, Goodman A et al. , 2016. Cognitive priming and cognitive training: immediate and far transfer to academic skills in children. Sci Rep 6, 32859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersema R, Van Der Meere J, Roeyers H, Van C, Baeyens D, 2006. Event rate and event-related potentials in ADHD. J Child Psychol Psychiatry 47(6), 560–567. [DOI] [PubMed] [Google Scholar]
- Wilcox RR, Keselman HJ, 2012. Modern regression methods that can substantially increase power and provide a more accurate understanding of associations. Eur J Personal 26(3), 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF, 2005. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry 57, 1336–1346. [DOI] [PubMed] [Google Scholar]
- Winsberg BG, Javitt DC, Silipo GS, Doneshka P, 1993. Mismatch negativity in hyperactive children: Effects of methylphenidate. Psychopharmacol Bull. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.