Figure 1.
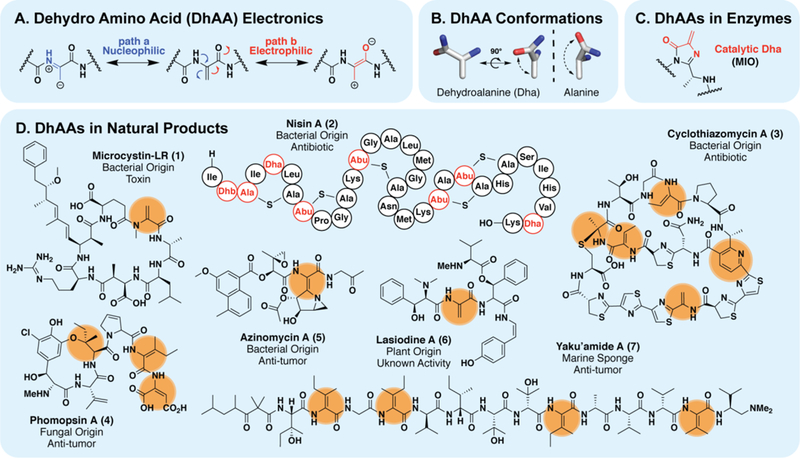
(A) Push-pull electronic structure of dhAAs makes them act as electrophiles or nucleophiles under given conditions. (B) dhAAs exhibit unusual conformational proclivity and restricted rotation relative to natural amino acids. (C) Some enzymes are armed with modified dhAAs that act as catalytic electrophiles to perform chemistry. (D) dhAAs are abundant in natural products from bacteria, fungi and plants. Additional chemistry can be performed on dhAAs to install more complex functionality, such as the pyridine ring in thiopeptides like cyclothiazomycin (3). The thioether and phenolic ether bonds, from cyclothiazomycin A (3) and phomopsin A (4) respectively, may also originate from dhAA modifications.
