Figure 5.
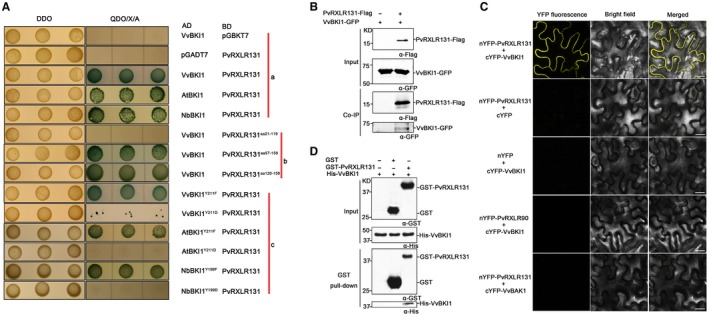
Confirmation of the interaction between PvRXLR131 and VvBKI1. (A) Y2H analysis illustrates the interaction between BKI1 and PvRXLR131. Yeast strain Y2H Gold containing both prey (AD) and bait (BD) can grow on DDO non‐selective medium. Colonies on DDO were drip inoculated on QDO selective medium supplemented with X‐α‐Gal and aureobasidin A (QDO/X/A); strains harbouring two interacting proteins can grow on QDO/X/A and turn blue. The empty vector pGBKT7(BD) or pGADT7(AD) was used as control. Group a demonstrated that PvRXLR131 interacted with VvBKI1, AtBKI1 and NbBKI1. The C‐terminal effector domain of PvRXLR131 was responsible for the interaction between VvBKI1 and PvRXLR131 (Group b). Mutagenesis of BKI1s at a conserved tyrosine site (VvBKI1Y211, AtBKI1 Y211 and NbBKI1Y199) into aspartic acid disrupted the interaction with PvRXLR131, while phenylalanine mutations did not (Group c). (B) Confirmation of the interaction using co‐immunoprecipitation (Co‐IP). VvBKI1‐GFP was transiently expressed alone or with PvRXLR131‐Flag in Nicotiana benthamiana. Total proteins were extracted and immunoprecipitated with anti‐Flag antibody. Extracted proteins and the bound protein were detected using immunoblot with the indicated antibodies. (C) Confirmation of the interaction using BiFC. The C‐terminus of YFP (cYFP) was fused to the N‐terminus of VvBKI1 and VvBAK1 (control), and the N‐terminus of YFP (nYFP) was fused to the N‐terminus of PvRXLR131 and PvRXLR90 (control); both of the indicated recombinant proteins were transiently co‐expressed in N. benthamiana. Fluorescence signals of YFP were observed at 2 dpi using confocal microscopy. Bars = 20 µm. (D) Confirmation of the interaction using GST pull‐down. His‐VvBKI1, GST‐PvRXLR131 and GST were affinity purified. GST pull‐down assay was performed for each combination as indicated. The amount of bound protein His‐VvBKI1 was analysed using anti‐His immunoblot.
