Figure 4.
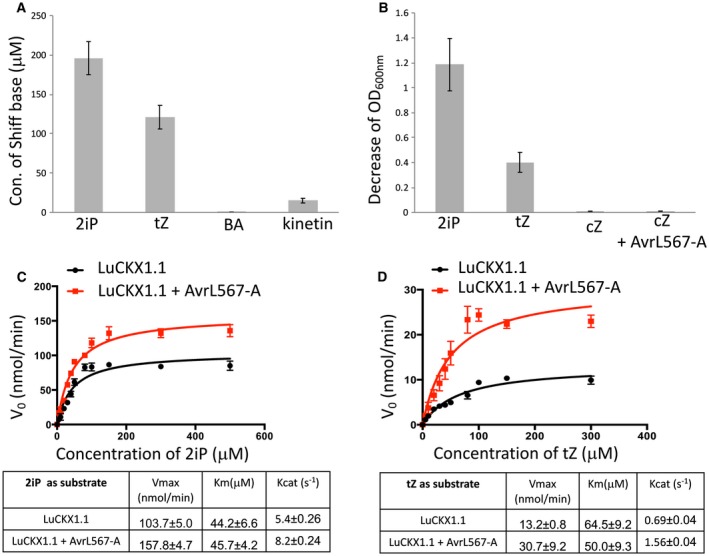
AvrL567‐A enhances LuCKX1.1 activity in vitro. (A) The concentrations of Schiff bases were measured in an endpoint assay to indicate the conversions of 2‐isopentenyladenine (2iP), trans‐zeatin (tZ), N6‐benzyladenine (BA) and N6‐furfuryladenine (kinetin) by LuCKX1.1. (B) The bleaching of 2,6‐dichlorophenolindophenol (DCPIP) due to reduction, as an electron acceptor in the LuCKX1.1‐mediated cytokinin oxidation, was measured in this assay to indicate the oxidative conversions of 2iP, tZ, cis‐zeatin (cZ) and cZ in the presence of AvrL567‐A. (C, D) Kinetic assays on LuCKX1.1 represented as Michaelis–Menten plots. The concentrations of substrates 2iP (C) and tZ (D) were plotted against the initial velocity of the enzymatic reaction (V 0). Error bars represent standard errors from three independent measurements. Black and red lines represent the non‐linear fits for these plots from which the enzymatic parameters were calculated. The figure was prepared using GraphPad Prism version 5.0. [Colour figure can be viewed at wileyonlinelibrary.com]
