Summary
It is hypothesized that the virulence of phytopathogenic fungi is mediated through the secretion of small effector proteins that interfere with the defence responses of the host plant. In Fusarium oxysporum, one family of effectors, the Secreted In Xylem (SIX) genes, has been identified. We sought to characterize the diversity and evolution of the SIX genes in the banana‐infecting lineages of F. oxysporum f. sp. cubense (Foc). Whole‐genome sequencing data were generated for the 23 genetic lineages of Foc, which were subsequently queried for the 14 known SIX genes (SIX1–SIX14). The sequences of the identified SIX genes were confirmed in a larger collection of Foc isolates. Genealogies were generated for each of the SIX genes identified in Foc to further investigate the evolution of the SIX genes in Foc. Within Foc, variation of the SIX gene profile, including the presence of specific SIX homologues, correlated with the pathogenic race structure of Foc. Furthermore, the topologies of the SIX gene trees were discordant with the topology of an infraspecies phylogeny inferred from EF‐1α/RPB1/RPB2 (translation elongation factor‐1α/RNA polymerase II subunit I/RNA polymerase II subunit II). A series of topological constraint models provided strong evidence for the horizontal transmission of SIX genes in Foc. The horizontal inheritance of pathogenicity genes in Foc counters previous assumptions that convergent evolution has driven the polyphyletic phylogeny of Foc. This work has significant implications for the management of Foc, including the improvement of diagnostics and breeding programmes.
Keywords: effectors, Fusarium oxysporum f. sp. cubense, horizontal gene transfer
Introduction
The collective host range of the Fusarium oxysporum species complex (FOSC) is remarkably diverse, encompassing not only plant species, but also animals as distantly related as arthropods and humans (Nucci and Annaisie, 2007; Ortoneda et al., 2004; Snyder and Hansen, 1940; Teetor‐Barsch and Roberts, 1983). In contrast with the broad host range of the species complex as a whole, each individual pathogenic isolate possesses the ability to infect just one or a few host species (Snyder and Hansen, 1940). Pathogenic isolates are conventionally grouped into infraspecific assemblages, known as formae speciales (ff. spp.), on the basis of the host species affected. However, this classification, which is based solely on the host‐specific pathogenicity of an isolate, is an informal taxonomic grouping and is often not phylogenetically informative (O'Donnell et al., 2013; Snyder and Hansen, 1940). Formae speciales are further divided into ‘races’ in which pathogenic variation exists in relation to different cultivars of the same host species. In this study, we have focused on the forma specialis (f. sp.) cubense, which includes all FOSC isolates pathogenic to Musa spp. (Snyder and Hansen, 1940). Significant pathogenic variation exists across this forma specialis with respect to various commercial cultivars, and it is therefore divided into four races (Stover, 1990; Stover and Buddenhagen, 1986; Stover and Simmonds, 1987). Race 1 is pathogenic to Gros Michel (AAA genome), Pisang Awak (ABB) and a range of other cultivars, primarily with the AAB genome (Stover, 1990; Stover and Buddenhagen, 1986; Stover and Simmonds, 1987); race 2 is pathogenic to race 1‐susceptible cultivars, as well as Bluggoe and other cultivars with the ABB genome (Stover, 1990; Stover and Buddenhagen, 1986; Stover and Simmonds, 1987); race 3 affects Heliconia species, not banana, and is therefore no longer considered to be part of the cubense race structure (Ploetz and Pegg, 2000; Waite, 1963); and race 4 is pathogenic to all race 1‐ and 2‐susceptible cultivars plus the Cavendish subgroup (AAA) (Stover and Simmonds, 1987; Su et al., 1986). The isolates within race 4 are commonly divided into two groups: ‘tropical race 4’ (TR4) and ‘subtropical race 4’ (STR4). TR4 is defined as those isolates which cause disease on Cavendish in tropical conditions, whereas STR4 is defined as those isolates which, in the absence of predisposing factors, are unable to infect Cavendish in the tropics, but do cause disease in subtropical conditions (Moore et al., 1993).
As an alternative to the race classification system, FOSC isolates can be characterized according to vegetative compatibility, which is the ability of an isolate to anastomose and form a stable heterokaryon (Moore et al., 1993; Ploetz and Correll, 1988; Puhalla, 1985). Isolates that are vegetatively compatible with one another are said to comprise a vegetative compatibility group (VCG) and typically share common biological, physiological and pathological traits (Caten and Jinks, 1966). Within F. oxysporum f. sp. cubense (Foc), 25 VCGs (0120–01224) have thus far been described (Bentley et al., 1995; Gerlach et al., 2000; Katan and Di Primo, 1999; Moore et al., 1993; Ploetz and Correll, 1988). However, VCG 0127 originally reported by Ploetz and Correll (1988) is no longer considered to be valid (Ploetz, 1994). Associations between VCGs and race are summarized in Table 1. Phylogenetic analysis of the various genetic lineages of Foc have repeatedly demonstrated that this forma specialis has a polyphyletic evolutionary history (Baayen et al., 2000; Fourie et al., 2009; Groenewald et al., 2006).
Table 1.
Race structure of Fusarium oxysporum f. sp. cubense by vegetative compatibility group (VCG) (Bentley et al., 1995, 1998; Gerlach et al., 2000; Groenewald et al., 2006; Jones, 2000; Katan and Di Primo, 1999; Moore et al., 1993; Ploetz, 1994; Ploetz and Correll, 1988).
| VCG | VCG complex | Race |
|---|---|---|
| 0120 | 0120–01215 | STR4 |
| 0121 | None | R4 * |
| 0122 | None | R4 * |
| 0123 | None | 1 |
| 0124 | 0124–0125–0128–01220 | 1, 2 |
| 0125 | 0124–0125–0128–01220 | 1, 2 |
| 0126 | None | R1/R4 † |
| 0127 | N/A | No longer valid ‡ |
| 0128 | 0124–0125–0128–01220 | 1, 2 |
| 0129 | 0129–01211 | STR4 |
| 01210 | None | 1 |
| 01211 | 0129–01211 | STR4 |
| 01212 | None | Undetermined |
| 01213 | 01213–01216 | TR4 § |
| 01214 | None | 2 |
| 01215 | 0120–01215 | STR4 |
| 01216 | 01213–01216 | TR4 § |
| 01217 | None | 1 |
| 01218 | None | 1 |
| 01219 | None | Undetermined |
| 01220 | 0124–0125–0128–01220 | 1 |
| 01221 | None | Undetermined |
| 01222 | None | Undetermined |
| 01223 | None | Undetermined |
| 01224 | None | Undetermined |
*The subclassification of VCGs 0121 and 0122 to either TR4 or STR4 is ambiguous as there are reports that these VCGs cause disease on Cavendish in tropical conditions; however, it has been observed that these VCGs are less aggressive than isolates of VCG 01213/16 (Buddenhagen, 2009; Moore et al., 1993; O'Neill et al., 2011).
†The race of VCG 0126 is ambiguous as isolates from VCG 0126 demonstrate many traits associated with race 4, such as the production of odoratum on medium and a close genetic relationship to other VCGs of race 4. However, there is limited evidence to suggest that it is capable of infecting Cavendish and it is commonly referred to as race 1 based on its host range (Pegg et al., 1994).
‡VCG 0127, originally reported by Ploetz and Correll (1988), is no longer considered to be valid (Ploetz, 1994).
§VCG 01213 and 01216 are now considered to be the same VCG complex (VCG 01213/16) (Bentley et al., 1998).
The molecular mechanisms underpinning the virulence of Foc are of great scientific interest as they relate to the applied management of Fusarium wilt and, more generally, to our understanding of the evolution of the host–pathogen interaction. Presently, the ‘zig–zag model’ is the accepted paradigm for the molecular interactions between phytopathogens and their hosts (Jones and Dangl, 2006). Briefly, under this model, conserved molecular patterns associated with microbial pathogens, such as flagellin or chitin, elicit an initial defence response from the host plant. Pathogens have evolved mechanisms to subvert this initial defence response, including the secretion of small effector proteins at the infection interface. Effector proteins are known to have a range of functions, including masking of the presence of the pathogen, suppression of host defence responses and transcriptional reprogramming of the host cell. Analogous to an arms race, plants have evolved classes of resistance genes that associate with and recognize these effectors and trigger a second wave of defence responses. Pathogens that are able to mitigate or suppress this secondary level of defence have a distinct selective advantage. Consequently, the genes coding for effectors are often under strong selection pressure to gain mutations in these genomic regions that result in a loss of recognition by the corresponding resistance proteins produced by the host. It has been demonstrated in multiple plant pathogens, including F. oxysporum, that the host range and specificity of an isolate are affected by the effectors in its molecular arsenal (Gawehns et al., 2014; Houterman et al., 2008, 2009; Ma et al., 2015; Rep et al., 2004, 2005).
In the phytopathogenic isolates of F. oxysporum, the Secreted In Xylem (SIX) genes form the only family of effectors that has been identified to date. In the tomato‐infecting forma specialis, F. oxysporum f. sp. lycopersici (Fol), 14 SIX genes (SIX1–SIX14) have been experimentally confirmed (Houterman et al., 2007; Lievens et al., 2009; Rep et al., 2004; Schmidt et al., 2013; Takken and Rep, 2010). Of these 14 genes, five Fol‐SIX genes (SIX1, SIX3, SIX4, SIX5 and SIX6) have been validated to encode genuine effector proteins in planta (Gawehns et al., 2014; Houterman et al., 2007; Lievens et al., 2009; Ma et al., 2015; Rep et al., 2004; Schmidt et al., 2013; Takken and Rep, 2010). The protein products of SIX1, SIX3/SIX5 and SIX4 are also avirulence factors as they are recognized by host defence or immune receptors (I‐3, I‐2 and I‐1, respectively) that have been introgressed into commercial cultivars of tomato (Houterman et al., 2008, 2009; Ma et al., 2015; Rep et al., 2004, 2005). Consistent with the zig–zag model, the emergence of new races in Fol is associated with either mutations in the coding region of the effector gene or complete effector gene loss events, which both result in the loss of recognition by the host immune receptors. Although the specific functions of the SIX proteins are unknown, some evidence indicates that the SIX proteins promote virulence through the manipulation of the hormone pathways and defence responses of the host (Gawehns et al., 2014; Ma et al., 2013, 2015; Thatcher et al., 2012).
Initially, the SIX genes were thought to be unique to Fol; however, numerous homologues have since been identified in other formae speciales, including betae, canariensis, cepae, ciceris, conglutinans, cubense, fragariae, lilii, lycopersici, medicaginis, melonis, niveum, passiflorae, pisi, radicis‐cucumerinum, radicis‐lycopersici, raphani, vasinfectum and zingiberi (Chakrabarti et al., 2011; Covey et al., 2014; Fraser‐Smith et al., 2014; Guo et al., 2014, Laurence et al., 2015; Lievens et al., 2009; Meldrum et al., 2012; Taylor et al., 2016; Thatcher et al., 2012; Williams et al., 2016). In Foc, homologues of SIX1, SIX2, SIX6, SIX7, SIX8, SIX9 and SIX13 have been identified (Fraser‐Smith et al., 2014; Guo et al., 2014; Meldrum et al., 2012; Taylor et al., 2016; Van Dam et al., 2016). However, research thus far has been limited to a narrow sample of the phylogenetic diversity of Foc.
In this study, we used a whole‐genome sequencing approach to screen isolates from Foc VCGs 0120 to 01223 for initial identification of homologues to SIX1 to SIX14. Subsequent polymerase chain reaction (PCR) analyses confirmed the distribution and diversity of the SIX genes established by the whole‐genome dataset. Gene tree analysis revealed that the SIX genes in Foc had an evolutionary history that was incongruent with the phylogeny of Foc established through nuclear genes conventionally used for phylogenetic reconstruction. We hypothesize that this incongruence is reflective of historical horizontal transfer of SIX genes between genetically distinct lineages of Foc.
Results
Variation in distribution and nucleotide sequence of SIX genes in VCGs of Foc
To initially identify SIX gene homologues in Foc, whole‐genome datasets were generated for 23 VCGs of Foc (n = 28); three F. oxysporum isolates from asymptomatic banana plants and five other formae speciales were queried for homologues of Fol‐SIX1 to Fol‐SIX14 (Table 2). In addition, the genomes of other F. oxysporum isolates available through public databases were also queried for homologues of Fol‐SIX1 to Fol‐SIX14 (Table S2, see Supporting Information). Seven SIX genes were identified in the VCGs of Foc: SIX1, SIX2, SIX6, SIX8, SIX9, SIX10 and SIX13. An orthologue of SIX4 was also identified in the VCGs of Foc; however, the start codon of SIX4 was non‐functional and is predicted to be a pseudogene. To further confirm the presence and sequence of the SIX gene homologues identified in the Foc whole‐genome datasets, a larger collection of Foc isolates was screened using PCR. The results from the analysis of the whole‐genome data and PCR screens are summarized in Table 3.
Table 2.
Fusarium oxysporum isolates used in this study, obtained as monoconidial cultures from the Department of Agriculture and Fisheries, Queensland Government, Australia; the Department of Primary Industry and Fisheries, Northern Territory Government, Australia; and the Agricultural Research Service Culture Collection, US Department of Agriculture.
| Forma specialis | VCG * | Accession † | Host | Geographical origin |
|---|---|---|---|---|
| cubense | 0120 | 44012 | Musa AAA ‘Cavendish’ | Australia |
| cubense | 0120 | 40182 | Musa AAA ‘Cavendish’ | Australia |
| cubense | 0120 | 58620 | Musa sp. (unidentified) | Indonesia |
| cubense | 0120 | 59164 | Musa AAA ‘Gros Michel’ | South Africa |
| cubense | 0120 | 58614 | Musa sp. (unidentified) | Canary Islands |
| cubense | 0121 | 62962 | Musa AA ‘Sucrier’ | Taiwan |
| cubense | 0121 | 62969 | Musa AAA ‘Cavendish’ | Taiwan |
| cubense | 0121 | 59104 | Musa sp. (unidentified) | Indonesia |
| cubense | 0121 | 58741 | Musa AAA ‘Cavendish’ | Malaysia |
| cubense | 0121 | 59084 | Musa AAA ‘Gros Michel’ | Indonesia |
| cubense | 0122 | 62892 | Musa AAA ‘Cavendish’ | Philippines |
| cubense | 0122 | 62901 | Musa AA ‘Pisang Lilin’ | Philippines |
| cubense | 0122 | 62894 | Musa AAA ‘Cavendish’ | Philippines |
| cubense | 0122 | 59154 | Musa AAA ‘Cavendish’ | Philippines |
| cubense | 0122 | 62893 | Musa AAA ‘Cavendish’ | Philippines |
| cubense | 0123 | 62895 | Musa AAB ‘Latundan’ | Philippines |
| cubense | 0123 | 59051 | Musa sp. (unidentified) | Indonesia |
| cubense | 0123 | 58737 | Musa sp. (unidentified) | Malaysia |
| cubense | 0123 | 58807 | Musa sp. (unidentified) | Thailand |
| cubense | 0123 | 58780 | Musa AAB ‘Latundan’ | Philippines |
| cubense | 0124 | 62933 | Musa sp. (unidentified) | Honduras |
| cubense | 0124 | 43997 | Musa AAB ‘Lady Finger’ | Australia |
| cubense | 0124 | 62911 | Musa sp. (unidentified) | India |
| cubense | 0124 | 58802 | Musa sp. (unidentified) | Thailand |
| cubense | 0124 | 62953 | Musa ABB ‘Bluggoe’ | Brazil |
| cubense | 0125 | 62957 | Musa sp. (unidentified) | India |
| cubense | 0125 | 44010 | Musa AAB ‘Lady Finger’ | Australia |
| cubense | 0125 | 62952 | Musa ABB ‘Bluggoe’ | Brazil |
| cubense | 0125 | 58692 | Musa ABB ‘Pisang Awak’ | Malaysia |
| cubense | 0125 | 58788 | Musa sp. (unidentified) | Thailand |
| cubense | 0126 | 59161 | Musa sp. (unidentified) | Papua New Guinea |
| cubense | 0126 | 59152 | Musa AAB ‘Latundan’ | Philippines |
| cubense | 0126 | 59046 | Musa sp. (unidentified) | Indonesia |
| cubense | 0126 | 58639 | Musa sp. (unidentified) | Indonesia |
| cubense | 0126 | 59060 | Musa sp. (unidentified) | Indonesia |
| cubense | 0128 | 22887 | Musa ABB ‘Bluggoe’ | Australia |
| cubense | 0128 | 44013 | Musa ABB ‘Bluggoe’ | Australia |
| cubense | 0128 | 44479 | Musa sp. (unidentified) | Australia |
| cubense | 0128 | 44616 | Musa sp. (unidentified) | Australia |
| cubense | 0128 | 44016 | Musa ABB ‘Bluggoe’ | Australia |
| cubense | 0129 | 40255 | Musa AAB ‘Lady Finger’ | Australia |
| cubense | 0129 | 42186 | Musa AAB ‘Lady Finger’ | Australia |
| cubense | 0129 | 63615 | Musa AAB ‘Lady Finger’ | Australia |
| cubense | 0129 | 44466 | Musa AAB ‘Lady Finger’ | Australia |
| cubense | 0129 | 63330 | Musa sp. (unidentified) | Australia |
| cubense | 01211 | 44073 | Musa sp. (unidentified) | Australia |
| cubense | 01211 | 39259 | Musa AAB ‘Lady Finger’ | Australia |
| cubense | 01210 | 26029 ‡ | Musa AAB ‘Silk’ | USA |
| cubense | 01212 | 62955 | Musa sp. (unidentified) | India |
| cubense | 01212 | 59037 | Musa sp. (unidentified) | India |
| cubense | 01213 | 40340 | Musa AAA ‘Cavendish’ | Australia |
| cubense | 01213 | 58734 | Musa AAA ‘Cavendish’ | Malaysia |
| cubense | 01213 | 58651 | Musa AAA ‘Cavendish’ | Indonesia |
| cubense | 01213 | 62560 | Musa AAA ‘Cavendish’ | Indonesia |
| cubense | 01214 | 25609 ‡ | Musa ABB ‘Harare’ | Malawi |
| cubense | 01214 | 36113 ‡ | Musa ABB ‘Bluggoe’ | Malawi |
| cubense | 01215 | 36112 ‡ | Musa AAA ‘Cavendish’ | South Africa |
| cubense | 01216 | 62779 | Musa AAA ‘Cavendish’ | Indonesia |
| cubense | 01216 | 59049 | Musa AAA ‘Cavendish’ | Indonesia |
| cubense | 01216 | 58697 | Musa AAA ‘Cavendish’ | Malaysia |
| cubense | 01216 | 58725 | Musa AAA ‘Cavendish’ | Malaysia |
| cubense | 01216 | 58746 | Musa AAA ‘Cavendish’ | Malaysia |
| cubense | 01217 | 58698 | Musa sp. (unidentified) | Malaysia |
| cubense | 01217 | 59147 | Musa sp. (unidentified) | Malaysia |
| cubense | 01217 | 58681 | Musa sp. (unidentified) | Malaysia |
| cubense | 01217 | 58683 | Musa sp. (unidentified) | Malaysia |
| cubense | 01217 | 58723 | Musa sp. (unidentified) | Malaysia |
| cubense | 01218 | 63259 | Musa sp. (unidentified) | Indonesia |
| cubense | 01218 | 58645 | Musa sp. (unidentified) | Indonesia |
| cubense | 01218 | 58700 | Musa sp. (unidentified) | Malaysia |
| cubense | 01218 | 58619 | Musa sp. (unidentified) | Indonesia |
| cubense | 01218 | 59041 | Musa sp. (unidentified) | Indonesia |
| cubense | 01219 | 58634 | Musa sp. (unidentified) | Indonesia |
| cubense | 01219 | 63261 | Musa sp. (unidentified) | Indonesia |
| cubense | 01219 | 58624 | Musa sp. (unidentified) | Indonesia |
| cubense | 01219 | 59115 | Musa BB ‘Pisang Kepok’ | Indonesia |
| cubense | 01219 | 58636 | Musa sp. (unidentified) | Indonesia |
| cubense | 01220 | 58803 | Musa sp. (unidentified) | Thailand |
| cubense | 01220 | 42103 | Musa AAA ‘Cavendish’ | Australia |
| cubense | 01221 | 36118 † | Musa ABB ‘Pisang Awak’ | Thailand |
| cubense | 01222 | 59170 | Musa sp. (unidentified) | Uganda |
| cubense | 01223 | 36116 ‡ | Musa AAB ‘Mysore’ | Malaysia |
| cubense | 0120/15 | 59028 | Musa AAA ‘Cavendish’ | Canary Islands |
| cubense | 0120/15 | 59052 | Musa sp. (unidentified) | Indonesia |
| cubense | 0120/15 | 62942 | Musa AAA ‘Cavendish’ | South Africa |
| cubense | 01213/16 | 59785 | Musa AAA ‘Cavendish’ | Australia |
| cubense | 01213/16 | 58821 | Musa AAA ‘Cavendish’ | Malaysia |
| cubense | 01213/16 | 59130 | Musa AAA ‘Cavendish’ | Indonesia |
| cubense | 0124/22 | 58813 | Musa sp. (unidentified) | Uganda |
| cubense | 0124/5 | 62950 | Musa sp. (unidentified) | China |
| cubense | 0124/5 | 58774 | Musa AAA ‘Gros Michel’ | Mexico |
| cubense | 0124/5 | 44080 | Musa AAB ‘Lady Finger’ | Australia |
| fragariae | N/A | 53860 | Fragaria × ananassa | Australia |
| medicaginis | N/A | 5189 | Medicago sativa | Australia |
| niveum | N/A | 36955 § | Citrullus sp. | Australia |
| passiflorae | N/A | 28044 | Passiflora edulis | Australia |
| zingiberi | N/A | 39299 | Zingiber officinale | Australia |
| Non‐path ¶ | N/A | 29093 | Musa sp. (unidentified) | Australia |
| Non‐path ¶ | N/A | 29094 | Musa sp. (unidentified) | Australia |
| Non‐path ¶ | N/A | 45952 | Musa sp. (unidentified) | Australia |
*VCG, vegetative compatibility group.
†BRIP accession code assigned by the Department of Agriculture and Fisheries, Queensland Government, Australia unless otherwise indicated.
‡NRRL accession code assigned by the Agricultural Research Service Culture Collection, US Department of Agriculture.
§Accession code assigned by the Department of Primary Industry and Fisheries, Northern Territory Government, Australia.
¶Putative non‐pathogen of banana (Forsyth et al., 2006).
Table 3.
Homologues of Secreted In Xylem (SIX) genes detected in isolates of Fusarium oxysporum f. sp. cubense. Single‐letter code indicates the sequence variant of a Foc‐SIX gene homologue detected in an associated isolate. A ‘–’ has been used to denote the absence of a SIX gene in the corresponding isolate.
| SIX gene | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Race | VCG * | Accession † | 1 | 2 | 3 | 4 ** | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| 1 | 0123 | 62895 | d, f § | – | – | b | – | b | – | – | a | – | – | – | a | – |
| 1 | 0123 | 59051 | f | – | b | b | – | – | a | – | a | |||||
| 1 | 0123 | 58737 | d, f | – | b | b | – | – | a | – | a | |||||
| 1 | 0123 | 58807 | d, f | – | b | b | – | – | a | – | a | |||||
| 1 | 01210 | 26029 ‡ | d, f | b ¶ | – | b | – | b | – | – | a | – | – | – | a | – |
| 1 | 01217 | 58698 | d, f | – | – | b | – | b | – | – | a | – | – | – | a | – |
| 1 | 01217 | 59147 | d, f | – | – | b | – | b | – | – | a | – | – | – | a | – |
| 1 | 01217 | 58681 | d | – | b | b | – | – | a | – | a | |||||
| 1 | 01217 | 58683 | d, f | – | b | b | – | – | a | – | d | |||||
| 1 | 01217 | 58723 | d, f | – | b | b | – | – | a | – | – | |||||
| 1 | 01218 | 63259 | d, f | – | – | b | – | b | – | – | a, b | – | – | – | a | – |
| 1 | 01218 | 58645 | d, f | – | b | b | – | – | a, b | – | a | |||||
| 1 | 01218 | 58700 | d, f | – | b | b | – | – | a, b | – | – | |||||
| 1 | 01218 | 58619 | d, f | – | b | b | – | – | a, b | – | a | |||||
| 1 | 01218 | 59041 | d, f | – | b | b | – | a, b | – | a | ||||||
| 1 | Unknown | N2 [Link] , [Link] | d, f | – | – | b | – | b | – | – | a | – | – | – | a | – |
| 1, 2 | 0124 | 62933 | f | – | – | b | – | b | – | – | a | – | – | – | a | – |
| 1, 2 | 0124 | 43997 | f | – | b | b | – | – | a | – | a | |||||
| 1, 2 | 0124 | 62911 | d, f | – | b | b | – | – | a | – | a, b | |||||
| 1, 2 | 0124 | 58802 | d, f | – | b | b | – | – | a | – | a | |||||
| 1, 2 | 0124 | 62953 | f | – | b | b | – | – | a | – | a | |||||
| 1, 2 | 0124/5 | 62950 | d, f | – | b | b | – | – | a | – | a | |||||
| 1, 2 | 0124/5 | 44080 | d, f | – | b | b | – | – | a | – | a | |||||
| 1, 2 | 0124/22 | 58813 | d, f | – | b | b | – | – | a | – | a | |||||
| 1, 2 | 0125 | 62957 | f | – | – | b | – | b | – | – | a | – | – | – | a | – |
| 1, 2 | 0125 | 44010 | f | – | b | b | – | – | a | – | a | |||||
| 1, 2 | 0125 | 62952 | d, f | – | b | b | – | – | a | – | a | |||||
| 1, 2 | 0125 | 58692 | d, f | – | b | b | – | – | a | – | a | |||||
| 1, 2 | 0125 | 58788 | d, f | – | b | b | – | – | a | – | a | |||||
| 1, 2 | 0128 | 22887 | f | – | – | b | – | b | – | – | a | – | – | – | – | – |
| 1, 2 | 0128 | 44013 | f | – | b | b | – | – | a | – | – | |||||
| 1, 2 | 0128 | 44479 | f | – | b | b | – | – | a | – | – | |||||
| 1, 2 | 0128 | 44616 | f | – | b | b | – | – | a | – | – | |||||
| 1, 2 | 0128 | 44016 | f | – | b | b | – | – | a | – | – | |||||
| 1, 2 | 01220 | 58803 | d, f | – | – | b | – | b | – | – | a | – | – | – | a | – |
| 1, 2 | 01220 | 42103 | f | – | b | b | – | – | a | – | a | |||||
| 2 | 01214 | 25609 ‡ | f | – | – | – | – | – | – | – | a, c | – | – | – | a | – |
| 2 | 01214 | 36113 ‡ | f | – | – | – | – | – | – | – | a, c | – | – | – | a | – |
| STR4 | 0120 | 44012 | g | d | – | a | – | – | a | a3, b | a | – | – | – | – | – |
| STR4 | 0120 | 40182 | g | d | a | – | a | a3, b | a | – | – | |||||
| STR4 | 0120 | 58620 | g | d | a | – | a | a3, b | a | – | – | |||||
| STR4 | 0120 | 59164 | g | d | a | – | a | a3, b | a | – | – | |||||
| STR4 | 0120 | 58614 | g | d | a | – | a | a3, b | a | – | – | |||||
| STR4 | 0120/15 | 59028 | g | d | a | – | a | a3, b | a | – | – | |||||
| STR4 | 0120/15 | 62942 | g | d | a | – | a | a3, b | a | – | – | |||||
| STR4 | 0126 | 59161 | g | c | – | a | – | – | a | a3, b | a | – | – | – | – | – |
| STR4 | 0126 | 59152 | g | c | a | – | a | a3, b | a | – | – | |||||
| STR4 | 0126 | 59046 | g | c | a | – | a | a3, b | a | – | – | |||||
| STR4 | 0126 | 58639 | g | c | a | – | a | a3, b | a | – | – | |||||
| STR4 | 0126 | 59060 | g | c | a | – | a | a3, b | a | – | – | |||||
| STR4 | 0129 | 40255 | g | c | – | a | – | – | a | a3, b | a | – | – | – | – | – |
| STR4 | 0129 | 42186 | g | c | – | a | – | – | a | a3, b | a | – | – | – | – | – |
| STR4 | 0129 | 63615 | g | c | a | – | a | a3, b | a | – | – | |||||
| STR4 | 0129 | 44466 | g | c | a | – | a | a3, b | a | – | – | |||||
| STR4 | 0129 | 63330 | g | c | a | – | a | a3, b | a | – | – | |||||
| STR4 | 01211 | 39259 | g | c | – | a | – | – | a | a3, b | a | – | – | – | – | – |
| STR4 | 01211 | 44073 | g | c | a | – | a | a3, b | a | – | – | |||||
| STR4 | 01215 | 36112 ‡ | g | d | – | c | – | – | a | a3, b | a | – | – | – | – | – |
| TR4 | 01213 | 40340 | a, h, i | a | – | c | – | a | – | a1, a2 | a | – | – | – | a, e | – |
| TR4 | 01213 | 62560 | a, h, i | a | – | c | – | a | – | a1, a2 | a | – | – | – | a, e | – |
| TR4 | 01213 | 58734 | a, h, i | a | c | a | – | a1, a2 | a | – | a, e | |||||
| TR4 | 01213 | 58651 | a, h, i | a | c | a | – | a1, a2 | a | – | a, e | |||||
| TR4 | 01213/16 | 59785 | a, h, i | a | c | a | – | a1, a2 | a | – | a, e | |||||
| TR4 | 01213/16 | 58821 | a, h, i | a | c | a | – | a1, a2 | a | – | a, e | |||||
| TR4 | 01213/16 | 59130 | a, h, i | a | c | a | – | a1, a2 | a | – | a, e | |||||
| TR4 | 01213/16 | 54006 [Link] , [Link] | a, h, i | a | – | c | – | a | – | a1 | a | – | – | – | a | – |
| TR4 | 01216 | 62779 | a, h, i | a | – | c | – | a | – | a1, a2 | a | – | – | – | a, e | – |
| TR4 | 01216 | 59049 | a, h, i | a | c | a | – | a1, a2 | a | – | e | |||||
| TR4 | 01216 | 58697 | a, h, i | a | c | a | – | a1 | a | – | a | |||||
| TR4 | 01216 | 58725 | a, h, i | a | c | a | – | a1, a2 | a | – | a, e | |||||
| TR4 | 01216 | 58746 | a, h, i | a | c | a | – | a1, a2 | a | – | a, e | |||||
| TR4 | Unknown | B2 [Link] , [Link] | a, h, i | a | – | c | – | a | – | a1 | a | – | – | – | a, e | – |
| R4 | 0121 | 62962 | b, h, i | a | – | c | – | a | b | a1, a2 | a | a | – | – | e | – |
| R4 | 0121 | 62969 | b, h, i | a | – | c | – | a | b | a1, a2 | a | a | – | – | e | – |
| R4 | 0121 | 59104 | b, h, i | a | c | a | b | a1, a2 | a | a | e | |||||
| R4 | 0121 | 58741 | b, h, i | a | c | a | b | a1, a2 | a | a | e | |||||
| R4 | 0121 | 59084 | b, h, i | a | c | a | b | a1, a2 | a | a | e | |||||
| R4 | 0122 | 62892 | c, i | – | – | – | – | – | – | a3 | a | – | – | – | c | – |
| R4 | 0122 | 62901 | c, i | – | – | – | – | a3 | a | – | c | |||||
| R4 | 0122 | 62894 | c, i | – | – | – | – | a3 | a | – | c | |||||
| R4 | 0122 | 59154 | c, i | – | – | – | – | a3 | a | – | c | |||||
| R4 | 0122 | 62893 | c, i | – | – | – | – | a3 | a | – | c | |||||
| UD | 01212 | 62955 | f | – | – | b | – | b | – | – | a | – | – | – | a | – |
| UD | 01212 | 59037 | f | – | b | b | – | – | a | – | a | |||||
| UD | 01219 | 58634 | g | c | – | a | – | – | a | a3, b | a | – | – | – | – | – |
| UD | 01219 | 63261 | g | c | a | – | a | a3, b | a | – | – | |||||
| UD | 01219 | 58624 | g | c | a | – | a | a3, b | a | – | – | |||||
| UD | 01219 | 59115 | g | c | a | – | a | a3, b | a | – | – | |||||
| UD | 01219 | 58636 | g | c | a | – | a | a3, b | a | – | – | |||||
| UD | 01221 | 36118 ‡ | e | – | – | – | – | – | – | – | a | – | – | – | a, d | – |
| UD | 01222 | 59170 | d, f | – | – | – | – | – | – | – | a | – | – | – | a | – |
| UD | 01223 | 36116 ‡ | d | – | – | b | – | b | – | – | a | – | – | – | a | – |
Whole‐genome datasets were initially analysed for Foc‐SIX gene homologues in the accessions that are shaded. Subsequent PCR analysis confirmed the presence and sequence of the Foc‐SIX gene homologues in all accessions.
*VCG, vegetative compatibility group.
†BRIP accession code assigned by the Department of Agriculture and Fisheries, Queensland Government, Australia unless otherwise indicated.
‡NRRL accession code assigned by the Agricultural Research Service Culture Collection, US Department of Agriculture.
§Coding sequence has been interrupted by a transposon with homology to an NHT‐2 retrotransposon (GenBank no. KP213325.1).
¶Predicted pseudogene as a result of a frameshift mutation.
**Predicted pseudogene as a result of a non‐function start codon.
††Chinese race 1 (N2) and race 4 isolate (B2) originally described by Guo et al. (2014).
‡‡Whole‐genome data were downloaded from the National Center for Biotechnology Information (NCBI) for analysis. The Foc‐SIX gene homologues in these accessions were not confirmed by polymerase chain reaction (PCR).
The SIX genes identified in the VCGs of races 1 and 2 were generally conserved both within a VCG and between the different VCGs (Table 3). The variation within the VCGs of races 1 and 2 was primarily observed within the SIX1 homologues. The VCGs of races 1 and 2 were distinguishable from the VCGs of race 4 by both the presence/absence of several SIX genes, such as SIX8, as well as differences in the Foc‐SIX gene homologues they carried. The VCGs 01212, 01221, 01222 and 01223 (all of an undetermined race) exhibited Foc‐SIX gene profiles that were most similar to VCGs known to be races 1 and 2 (Table 3).
In contrast with the conserved combination of Foc‐SIX genes identified in the VCGs of races 1 and 2, those Foc VCGs that were assigned to race 4 exhibited a more variable Foc‐SIX gene profile. Despite their shared host range, the VCGs of STR4 (VCGs 0120, 0126, 0129, 01211 and 01215), VCG 0121, VCG 0122 and TR4 (VCG 01213, 01213/16, 01216) were each shown to carry a unique combination of Foc‐SIX genes.
Of the seven SIX genes detected in the VCGs of Foc, only SIX1 and SIX9 were identified in all isolates of Foc. Interestingly, these two genes demonstrated different degrees of genetic diversity; nine distinct Foc‐SIX1 homologues were identified across the VCGs of Foc, whereas a single identical SIX9a homologue was identified in all isolates of Foc (Table 3).
It was also shown that the other formae speciales screened in this study did not share the same combination of SIX genes as any of the Foc VCGs (Table 4), nor were any of the sequences identified in other formae speciales identical to the Foc‐SIX gene homologues. Of particular interest was the lack of any SIX genes in any of the isolates of F. oxysporum isolated from asymptomatic banana plants (Table 4).
Table 4.
Fusarium oxysporum Secreted In Xylem (SIX) gene profile by forma specialis.
| SIX gene | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Forma specialis | Accession | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| Arabidopsis‐infecting (Arabidopsis Fo5176) | 5176 * | a | – | – | a, b § | – | – | – | a | a | – | – | – | – | – |
| conglutinans (Focg) | 54008 † | a | – | – | a, b § | – | – | – | a | – | – | – | – | – | – |
| fragariae (Fof) | 53860 * | a, b, c | – | – | – | – | – | – | – | – | – | – | – | a | – |
| Human‐infecting (Fosc 3a) | 32931 † | – | – | – | – | – | – | – | a ¶ | – | – | – | – | – | – |
| lycopersici (4287) (Fol 4287) | 34936 † | a | a | a | – | a | a | a | a, b | a | a | a | a | a | a |
| lycopersici (Fol MN25) | 54003 † | a | a | a | – | a | a | a | a | a | a | a | a | a | a |
| medicaginis (Fomg) | 5189 * | a, b, c ** | – | – | – | – | – | – | a | – | – | – | – | a, b | – |
| melonis (Fom) | 26406 † | a | – | – | – | – | a | – | – | – | – | a | – | a | – |
| niveum (Fon) | 36955 ‡ | a †† | – | – | a | – | a | – | a, b | a | – | a | – | a, b | – |
| passiflorae (Fopf) | 28044 * | – | – | – | – | – | a | – | a | a, b ‡‡ | – | a | – | – | – |
| pisi (Fop) | 37622 † | a, b | – | – | – | – | – | – | – | – | – | – | – | a | a |
| radicis‐lycopersici (Forl) | 26381 † | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| raphani (For) | 54005 † | – | – | – | – | – | – | – | a | a, b, c, d | – | – | – | – | – |
| Soil biocontrol (Fo 47) | 54002 † | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| vasinfectum (Fov) | 25433 † | – | – | – | – | – | – | – | – | a, b | – | – | – | a | – |
| zingiberi (Foz) | 39299 * | – | – | – | – | – | – | a | – | a | a | a | – | ||
| Non‐pathogen (Fo 29093) | 29093 * | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Non‐pathogen (Fo 29094) | 29094 * | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Non‐pathogen (Fo 45952) | 45952 * | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
Single‐letter code indicates the SIX gene homologue(s) detected in a particular isolate. SIX gene homologue designations correspond to the homologues identified within an individual forma specialis. A ‘–’ has been used to denote the absence of a SIX gene in the corresponding isolate. Abbreviations of the isolates are shown in parentheses.
*Accession codes are BRIP codes assigned by the Department of Agriculture and Fisheries, Queensland Government, Australia.
†Accession codes are NRRL codes assigned by the Agricultural Research Service Culture Collection, US Department of Agriculture.
‡Accession code assigned by the Department of Primary Industry and Fisheries, Northern Government, Australia.
§Pseudogene as a result of an incomplete coding sequence.
¶Incomplete coding sequence. Gene located at the end of a contig and no overlapping contigs could be found.
**Incomplete coding sequence.
††Incomplete coding sequence interrupted by a sequence with homology to a long terminal repeat (LTR) retrotransposon.
‡‡Incomplete coding sequence.
Discordance between the evolutionary history of the Foc‐SIX genes and housekeeping genes
In order to determine whether the evolutionary history of SIX genes in Foc was consistent with the infraspecies evolution of Foc, we initially established the infraspecies relationships of the F. oxysporum isolates in this study for which whole‐genome sequence information was available. A combined gene dataset comprising the translation elongation factor (EF‐1α) and two of the large RNA polymerase II subunits (RPB1 and RPB2) was employed to generate phylogenetic trees using maximum likelihood (ML), Bayesian inference (BI) and maximum parsimony (MP) as tree building methods. The resulting trees produced by the methods all recovered very similar topologies with similar clade support. The VCGs of Foc demonstrated polyphyletic relationships across the phylogenetic tree (Fig. 1). The VCGs of races 1 and 2, the VCGs 01212, 01221, 01222 and 01223 of Foc (all of undetermined race) as well as all other isolates of F. oxysporum were placed in clade A of the phylogenetic tree. The VCGs of TR4, STR4, VCG 01219 (undetermined race) and VCG 01210 were placed in clade B (Fig. 1). The placement of VCG 01210 with the VCGs of race 4, rather than the VCGs of race 1/race 2, was particularly intriguing, given that the SIX genes identified in VCG 01210 were almost identical to those of other VCGs of race 1 (Table 3; Fig. 1).
Figure 1.
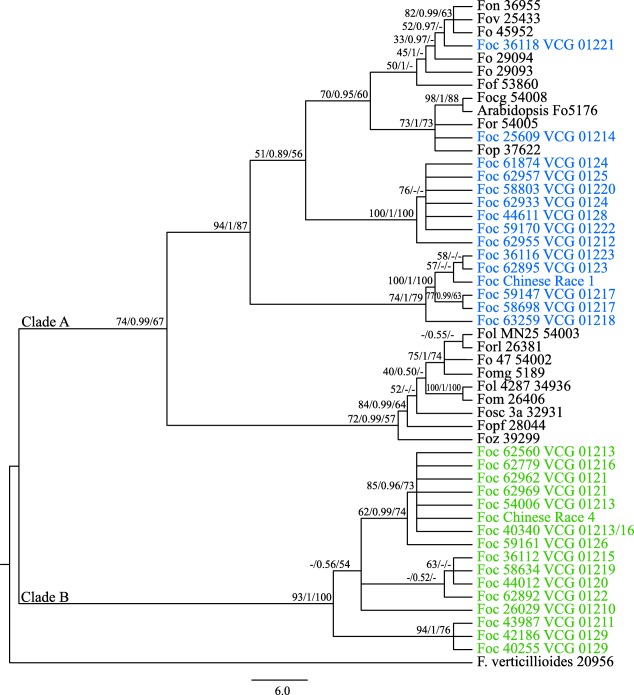
Best infraspecies phylogenetic tree inferred using maximum likelihood (ML) from the concatenated datasets of translation elongation factor‐1α (EF‐1α), RNA polymerase II subunit I (RPB1) and RNA polymerase II subunit II (RPB2) identified in isolates of Fusarium oxysporum used in this study. Trees with similar topologies were also inferred using maximum parsimony (MP) and Bayesian interference (BI) methods. Internal node support is indicated as ML bootstrap proportions/Bayesian probabilities/MP bootstrap proportions. The two major clades are as indicated. For each isolate, the abbreviated forma specialis and accession code as defined in Tables 3 and 4 are indicated. The isolates of F. oxysporum f. sp. cubense (Foc) are also labelled with their respective vegetative compatibility group (VCG). The isolates of Foc in clade A are shown in blue, whereas the isolates of Foc in clade B are shown in green. The other formae speciales and F. verticillioides NRRL 20956 are shown in black. Nucleotide sequences from F. verticillioides served as an outgroup to root the tree.
To further investigate the evolution of the SIX genes in Foc, we generated gene trees for each of the SIX genes present in Foc (Fig. 2). Each of the phylogenetic methods used in this process generated similar topologies for each of the SIX genes, with the exception of SIX1. The SIX gene sequences identified within the VCGs of Foc generally formed close sister clades to one another, although exceptions to this trend were observed in the gene trees of SIX1, SIX9 and SIX13. In the SIX1 gene tree, all tree‐building methods generated a tree in which the Foc‐SIX1a–g homologues formed a monophyletic group, whereas Foc‐SIX1i was placed in a separate sister lineage with the SIX1 homologues of some other formae speciales, most notably the SIX1 sequences identified in F. oxysporum f. sp. fragariae (Fig. 2a). The best tree recovered from the ML search method placed the Foc‐SIX1h homologue in close association with SIX1 homologues identified in other formae speciales (Fig. 2a). However, the best trees recovered using MP and BI methods recovered a tree that placed Foc‐SIX1h with the Foc‐SIX1a–g homologues. The placement of the Foc‐SIX1h clade remains unresolved, as all three phylogenetic methods did not recover high support values for the placement of the Foc‐SIX1h clade. The Foc‐SIX9 sequences also demonstrated very close relationships; however, a SIX9 sequence identified in raphanin was shown to be highly similar to Foc‐SIX9a and was found to be clustered in the same clade (Fig. 2f). The Foc‐SIX13a and Foc‐SIX13b homologues formed a monophyletic group, whereas Foc‐SIX13d and Foc‐SIX13e clustered into a distinct clade with the SIX13 homologues identified in other formae speciales (Fig. 2h). The polyphyletic distribution of the VCGs of Foc, as determined by housekeeping genes, contrasted strongly with the topologies of the SIX gene trees, in which most of the Foc‐SIX gene homologues formed close sister clades to one another.
Figure 2.
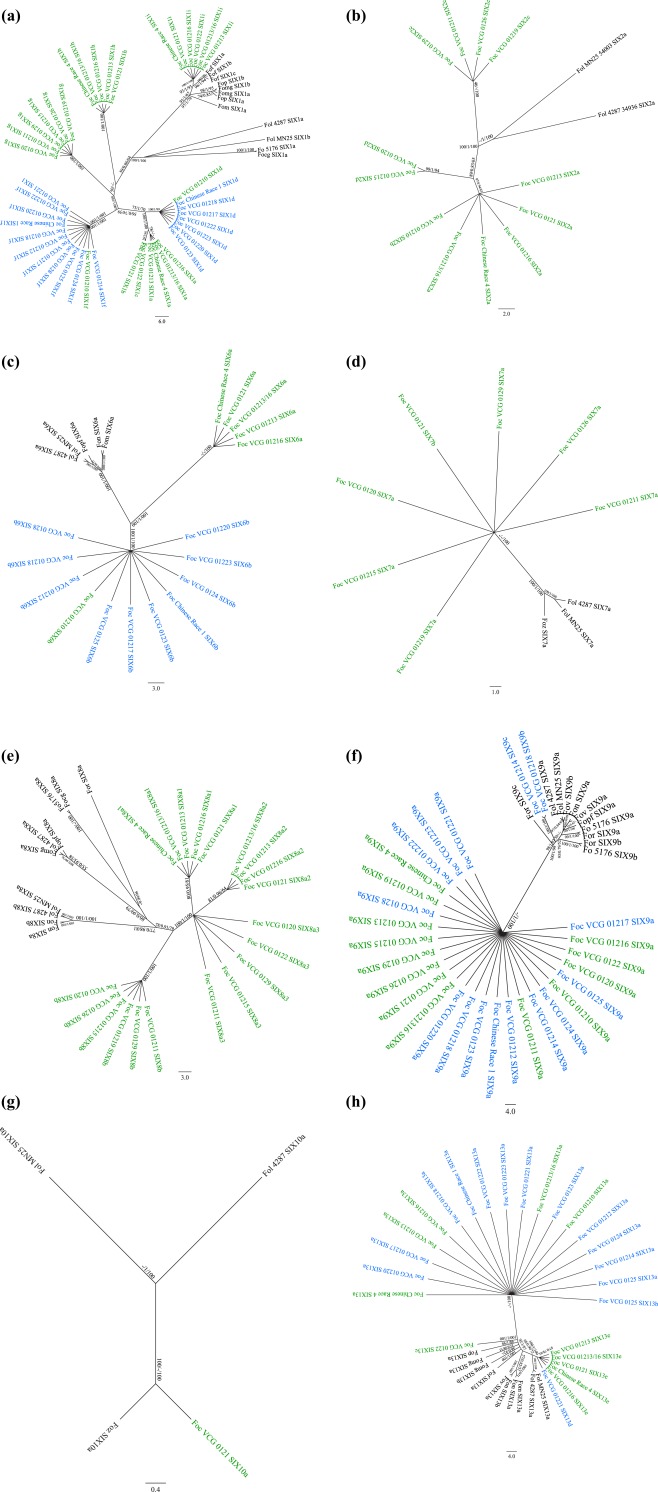
Best genealogies of the Secreted In Xylem (SIX) genes SIX1 (a), SIX2 (b), SIX6 (c), SIX7 (d), SIX8 (e), SIX9 (f), SIX10 (g) and SIX13 (h) generated using the maximum likelihood (ML) method. Trees with similar topologies were also inferred using maximum parsimony (MP) and Bayesian interference (BI) methods. Internal node support is indicated as ML bootstrap proportions/Bayesian probabilities/MP bootstrap proportions. For each external node, the abbreviated forma specialis as defined in Tables 3 and 4 in which the sequence was identified and the SIX gene homologue variant are indicated. For Fusarium oxysporum f. sp. cubense (Foc), the vegetative compatibility group (VCG) in which the SIX gene homologue was identified is also indicated. The VCGs of Foc that clustered in clade A of the infraspecies phylogeny are shown in blue, whereas the VCGs of Foc that clustered in clade B of the infraspecies phylogeny are shown in green. The sequences from other formae speciales are shown in black.
Evidence for the horizontal inheritance of SIX genes in the VCGs of Foc
As a result of the discordance between the infraspecies phylogeny and the genealogies of the SIX genes, we further investigated the evolutionary history of the SIX genes in Foc through a series of topological constraint tests. The incongruence between the infraspecies phylogeny and the SIX genes primarily occurred for the datasets in which Foc‐SIX gene homologues were identified in isolates occurring in both clade A and clade B of the infraspecies phylogeny. Therefore, topological constraint analyses were conducted only with the SIX genes identified in the VCGs of Foc from both clade A and clade B of the infraspecies phylogeny. To model vertical inheritance of the SIX genes in Foc, an ‘infraspecies constraint’ was implemented to force all taxa in the included SIX gene datasets to resemble the backbone of the infraspecies phylogeny, as determined previously using the combined EF‐1α/RPB1/RPB2 (Fig. 3a). With the exception of SIX9, all infraspecies constrained trees were found to have significantly worse tree scores when compared with the unconstrained gene trees (P < 0.05) (Table 5). Thus, this constraint analysis rejected the hypothesis of vertical inheritance of SIX1, SIX6 and SIX13 in Foc, whereas, surprisingly, the constraint analysis did not find a significant difference between the constrained and unconstrained trees for SIX9. A closer inspection of the best‐performing ML tree produced for SIX9 under the infraspecies constraint revealed that the gene tree had placed the SIX9 sequences identified in TR4, STR4, VCG 0126, VCG 01210 and VCG 01219 into a monophyletic ingroup to the other SIX9 sequences identified in Foc. Therefore, even under the infraspecies constraint, the best‐performing tree produced placed the SIX9 sequences of Foc into a close, monophyletic relationship, resulting in a constrained gene tree with a topology that was not significantly different from the unconstrained gene tree of SIX9 (Table 5).
Figure 3.
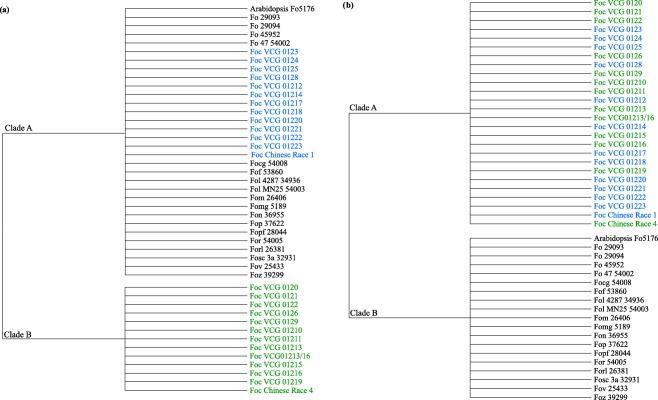
A visual representation of the models implemented for the topological constraint testing of the Secreted In Xylem (SIX) gene trees using an infraspecies backbone constraint model (a) and a Fusarium oxysporum f. sp. cubense (Foc) monophyletic constraint model (b). The infraspecies model constrained the SIX gene sequences identified in the vegetative compatibility groups (VCGs) of Foc and other formae speciales into the two major clades identified in the infraspecies phylogeny (Fig. 1). For each isolate, the abbreviated forma specialis and accession code as defined in Tables 3 and 4 are indicated. Under the Foc monophyletic model, the SIX gene sequences identified in the VCGs of Foc were constrained into a monophyletic clade, whereas the SIX gene sequences identified in other formae speciales were constrained into a reciprocating sister clade.
Table 5.
Results of topological constraint testing and tree score analysis using the maximum likelihood criterion.
| Constraint analysis | Difference in PSLL scores | AU | SH |
|---|---|---|---|
| Species constraint | |||
| SIX1 | 240.5 | <0.01 * | <0.01 * |
| SIX6 | 14.3 | 0.05 * | 0.01 * |
| SIX9 | 0.1 | 0.231 | 0.222 |
| SIX13 | 207.3 | <0.01 * | <0.01 * |
| Strict monophyly constraint | |||
| SIX1 | 41.8 | <0.01 * | <0.01 * |
| SIX6 | 0.1 | 0.194 | 0.173 |
| SIX9 | 13.9 | <0.05 * | <0.05 * |
| SIX13 | 47.5 | <0.01 * | <0.01 * |
| Relaxed monophyly constraint | |||
| SIX1 | 0.1 | 0.313 | 0.302 |
| SIX9 | 0.1 | 0.241 | 0.216 |
| SIX13 | 0.1 | 0.299 | 0.294 |
The Secreted In Xylem (SIX) gene datasets included in the analysis were constrained according to a species or strict monophyly topological constraint. The resulting tree scores from the best trees were compared for significant differences from the tree score of the unconstrained trees using the Approximately Unbiased (AU) and Shimodaira–Hasegawa (SH) tests. PSLL, per site log likelihood score.
*Significant at P < 0.05.
To model horizontal inheritance of the SIX genes in Foc, a ‘strict Foc‐SIX monophyly constraint’ forced the homologues of all Foc‐SIX sequences into a monophyletic clade, whereas the SIX homologues from other formae speciales formed a monophyletic sister clade (Fig. 3b). Under the strict clade constraint, the tree score for SIX6 was not significantly different from the tree scores of the respective unconstrained trees (P > 0.05) (Table 5). However, the tree scores of SIX1, SIX9 and SIX13 were significantly worse than their respective unconstrained trees (P < 0.05). The unconstrained trees of SIX1, SIX9 and SIX13 demonstrated that a subset of the Foc‐SIX gene homologues formed well‐supported ingroups with SIX homologues from other formae speciales (Fig. 2a,f,h). Specifically, Foc‐SIX1i was shown to cluster with the sequences of Fof‐SIX1, whereas Foc‐SIX13d and Foc‐SIX13e clustered with SIX13 homologues identified in Fof, Fol, Fom, Fomg, Fon, Fop and Fov. Furthermore, the unconstrained tree of SIX9 showed that Foc‐SIX9a was more similar to the For‐SIX9c homologue than it was to Foc‐SIX9b and Foc‐SIX9c. We hypothesized that the strict monophyly clade constraint imposed on the SIX1, SIX9 and SIX13 gene trees significantly affected the tree scores as a result of the placement of Foc‐SIX1i, For‐SIX9c, Foc‐SIX13c, Foc‐SIX13d and Foc‐SIX13e, respectively. We tested this hypothesis through a ‘relaxed Foc‐SIX monophyly constraint’ in which most of the Foc‐SIX homologues and the SIX homologues from other formae speciales were again placed into reciprocating sister polytomies. However, the relaxed Foc‐SIX clade constraint permitted the Foc‐SIX1i, For‐SIX9c, Foc‐SIX13d and Foc‐SIX13e homologues to freely sort across the constrained tree topology. Under the relaxed clade constraint, the tree scores of SIX1, SIX9 and SIX13 were not significantly different from the respective unconstrained gene trees (P > 0.05) (Table 5). The Foc‐SIX clade constraint analyses of the Foc‐SIX genes support the hypothesis of horizontal gene transfer of the SIX genes in Foc. Our results indicate that the role of horizontal gene transfer in the evolution of the SIX genes in Foc is complicated and dynamic.
Discussion
In the Fol–tomato pathosystem, changes in the cultivar‐specific pathogenicity of Fol are associated with mutations in the SIX genes that are recognized by host resistance genes (Houterman et al., 2008, 2009; Rep et al., 2004). These mutations in the corresponding SIX genes lead to a loss of recognition by the host and thus a gain of virulence phenotype in Fol. Consequently, the hypothesis of the current study was that the differences in pathogenicity of the races of Foc to banana cultivars may be reflected in the presence of effectors carried by each race. We have demonstrated that, within the forma specialis of Foc, there is variation in the distribution and diversity of the SIX genes.
The gene tree analysis revealed that the SIX genes in Foc have been both vertically and horizontally inherited in the lineages of Foc. The VCGs of Foc with the same host range, particularly the VCGs of races 1 and 2, share a highly conserved combination of SIX genes in demographically diverse lineages. In contrast, the SIX gene haplotypes for the VCGs of race 4 are distinct from the VCGs of races 1 and 2. We hypothesize that the highly conserved SIX gene haplotypes within a VCG and/or race is a result of the vertical inheritance of the SIX genes in these genetic lineages of Foc. A particularly interesting case of gene conservation was observed for the pseudogene, Foc‐SIX4. Despite not being predicted to code a functional protein, Foc‐SIX4 was identified in many lineages of Foc and the nucleotide sequence of SIX4 within the VCGs of Foc was highly conserved (percentage sequence similarity). In three of the genome assemblies of Foc, SIX4 and SIX6 are in close proximity to one another (∼500 bp). It is possible that the retention and conservation of SIX4 in Foc are a result of a linkage effect from a functional copy of SIX6. Another interesting observation was the identification of the same Foc‐SIX9a homologue in all VCGs of Foc screened in this study. Currently, the role of SIX9 in F. oxysporum during the infection of a host has not been investigated. This study makes SIX9 an excellent candidate for further functional validation.
The gene trees also revealed that several SIX genes common to the VCGs of races 1, 2 and 4 shared a common ancestor. Many studies have repeatedly demonstrated that the genes conventionally used to infer the genetic relationships of Foc are poorly correlated with the host‐specific pathogenicity of this pathogen and, as a result, the genetic lineages of Foc exhibit polyphyletic relationships (Baayen et al., 2000; Bentley et al., 1995, 1998; Boehm et al., 1994; Fourie et al., 2009; Koenig et al., 1997; O'Donnell et al., 1998, 2013). It has been hypothesized previously that the polyphyletic phylogeny of Foc is a result of the independent evolution of pathogenicity to banana in multiple lineages of F. oxysporum (Fourie et al., 2009; O'Donnell et al., 1998). However, we now hypothesize that the horizontal transfer of pathogenicity‐determining factors, such as the SIX genes, may have driven the polyphyletic evolution of Foc. The modelling of different inheritance patterns supported the horizontal acquisition of these genes in the genetically distinct lineages of Foc. Currently, we propose that a horizontal transfer event of a subset of SIX genes occurred between a race 1 or 2 donor, and the recipient was an ancestor of the race 4 lineages. We also hypothesize that the ancestor of the race 4 lineages either already harboured SIX genes prior to this transfer or was perhaps the recipient in further horizontal transfer events of SIX genes, and thus acquired the race 4‐specific homologues. Evidence to support this hypothesis was observed in the gene trees, which revealed that race 4‐specific homologues, such as Foc‐SIX1i and Foc‐SIX13e, clustered with SIX homologues identified in other formae speciales. Alternatively, each of these genes was identified as being multicopy, especially in the TR4 VCG 01213/16. An alternative hypothesis to horizontal gene transfer is that gene duplication has allowed the sequences of these homologues to significantly diverge and produce homoplastic gene trees. Further studies, including additional whole‐genome sequencing and assembly, will allow us to further investigate these hypotheses. Further evidence for horizontal gene transfer events with other formae speciales was observed for Foc‐SIX9a, which formed a close sister polytomy to the For‐SIX9c homologue. We hypothesize that these homologues have been involved in horizontal gene transfer; however, the directionality of the transfer is unknown, i.e. it is unclear whether Foc is the recipient or donor of these SIX genes. Evidence for the horizontal transfer of SIX genes has been reported in several formae speciales, including the Australian lineages of the cotton‐infecting F. oxysporum f. sp. vasinfectum, Arabidopsis‐infecting Fo5176 isolate and F. oxysporum f. sp. canariensis [causal agent of wilting in the Canary Island date palm (Phoenix canariensis)] (Chakrabarti et al., 2011; Laurence et al., 2015; Thatcher et al., 2012).
The underlying assumption for the hypothesis of horizontal transfer is that Foc also possesses small accessory chromosomes conferring pathogenicity that have been involved in horizontal transfer events, in a similar manner to Fol. Ma et al. (2010) demonstrated that a small accessory chromosome harbouring most of the SIX genes could be horizontally transferred from Fol to a genetically distinct isolate of F. oxysporum through the co‐incubation of the two isolates. This horizontal transfer event conferred a gain‐of‐pathogenicity towards tomato in the recipient strain. Fol is also a polyphyletic forma specialis and it is hypothesized that the polyphyletic relationships of the Fol isolates have arisen as a result of horizontal chromosome transfer events. Currently, the available genomes for Foc are highly fragmented and it is unclear whether the Foc‐SIX genes reside on one or multiple chromosomes. It is also unclear whether Foc carries its own, unique accessory chromosomes as reported for Fol. An improved genome assembly for the small accessory chromosomes of Foc would greatly improve our understanding of the genomic organization and evolution of this pathogen.
The horizontal transfer of accessory chromosomes carrying pathogenicity genes between the lineages of Foc has important implications for the biosecurity and disease management of Fusarium wilt in bananas. The recent detection of TR4 (VCG 01213/16) in Africa, Australia, Jordan, Lebanon and Pakistan has demonstrated the importance of the rapid and reliable identification of the causal organism during the response to a disease incursion (ABGC, 2015; Garcia‐Bastidas et al., 2014; IPPC, 2013; Ordonez et al. 2016; Syed et al., 2015). The horizontal movement of chromosomes harbouring the genes conferring the host‐ and cultivar‐specific pathogenicity of Foc could produce novel pathotypes of Foc that would not be identified with a conventional molecular diagnostic based on vertically inherited housekeeping genes. As a consequence, the molecular identification of Foc should include genes that can be inherited vertically through asexual reproduction, as well as genes inherited through horizontal chromosome transfer events, such as the SIX genes.
Currently, it is not known whether the Foc SIX proteins also facilitate the infection of banana plants as has been demonstrated in the tomato and Arabidopsis pathosystems (Gawehns et al., 2014; Houterman et al., 2008, 2009; Ma et al., 2015; Rep et al., 2004, 2005; Thatcher et al., 2012). Further studies, such as gene silencing and complementation, are required to validate the SIX proteins as effectors in the Foc–banana pathosystem. Exciting new technology, such as genome editing with the Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) with Cas9 system, offers a new approach for genetic knock‐outs and the incredibly accurate manipulation of target genes. Furthermore, our current understanding of effectors in F. oxysporum has been dictated by the initial studies completed in the Fol–tomato pathosystem. However, if we consider the evolutionary distance separating tomato and banana, and also hypothesize that fungal effector proteins facilitate the host‐specific pathogenicity of F. oxysporum, it should be expected that novel and undiscovered effectors remain to be identified in the lineages of Foc. The ongoing development of innovative solutions to this significant pathogen of banana is contingent on developing an improved understanding of pathogen evolution and functional molecular biology.
Experimental Procedures
Fusarium isolates and culture
Monoconidial isolates of F. oxysporum f. sp. cubense (VCGs 0120–01223), fragariae, medicaginis, niveum, passiflorae and zingiberi, and three putatively non‐pathogenic endophytes of banana were analysed in this study (Table 2). Isolates were cultured on half‐strength potato dextrose agar Difco (Becton, Dickinson and Co., Sparks, MD, USA) for 7 days at 25 ºC, after which the mycelium was harvested and stored at −80 ºC. Total cellular DNA was extracted using the BioSprint 15 DNA Plant Kit (Qiagen Pty. Ltd., Hilden, Germany) on a BioSprint 15 workstation (Qiagen Pty. Ltd., Hilden, Germany).
Next‐generation sequencing (NGS)
DNA libraries with insert sizes of c. 400 bp were created using the Nextera XT DNA Sample Preparation Kit (Illumina Inc., San Diego, CA, USA) according to the manufacturer's instructions. The Illumina MiSeq platform was used to generate paired‐end, 150‐bp sequence reads which were subsequently imported into Geneious v. 6.1.8 (http://www.geneious.com; Kearse et al., 2012) for analysis.
Identification of SIX genes in the genomes of F. oxysporum
Sequence reads from each isolate were mapped to reference sequences for SIX1–SIX14 within Geneious v. 6.1.8 (Table S1, see Supporting Information) using the default parameters associated with medium sensitivity mapping. Under these parameters, the mapping was allowed to iteratively search the datasets up to five times. Reads that mapped equally well to multiple sites in the reference were excluded from the mapping analysis. These constructs were manually inspected and used to generate consensus sequences, which were deposited into GenBank under the accessions KX434886–KX435052. When multiple homologues were detected in a single isolate, we manually curated a consensus sequence for each individual homologue, using the single nucleotide polymorphism (SNP) variants and paired reads to distinguish between each homologue. Multiple sequence alignments were then created for each SIX gene using the ClustalW interface within Geneious v. 6.1.8. In order to provide context for the diversity of SIX genes in F. oxysporum, SIX gene sequence data from the whole genomes of F. oxysporum publically available on GenBank were incorporated into the multiple sequence alignments. The details of the genomes included in the analysis are provided in Table S2.
PCR analysis of SIX genes in Foc
A larger collection of 89 Foc isolates representing all VCGs and including the isolates in the NGS panel were screened for the Foc‐SIX genes using PCR (Table S3, see Supporting Information). Primers were designed both manually and using Primer3 to amplify each of the Foc‐SIX genes identified from whole‐genome sequencing analysis. The primers and respective thermocycling conditions for SIX gene amplification are given in Table S3. PCR amplifications were performed in a Applied Biosystems Thermo Fisher Scientific (Singapore) thermocycler in 25 µL reactions consisting of 12.5 μL of GoTaq Green Master Mix (Promega, Madison, WI, USA), 1 μL of forward primer (10 μm; Integrated DNA Technologies Inc., Singapore), 1 μL of reverse primer (10 μm; Integrated DNA Technologies, Inc.), 1 μL of template DNA and 9.5 μL of nuclease‐free water. The results of the PCR amplifications were visualized by electrophoresis on a 1% agarose gel stained with ethidium bromide on a UV transilluminator. Amplicons were purified using the Wizard SV PCR and Gel Clean‐Up kit (Promega), and sequenced in the forward and reverse directions using the PCR amplification primers in a single‐pass Sanger sequencing reaction by Macrogen (Seoul, South Korea). In the instances in which multiple amplicons were identified from initial sequencing for SIX1 and could not be selectively amplified using PCR, the amplicons were cloned using the pCR2.1 vector with the TOPO TA kit (Invitrogen Life Technologies, Carlsbad, CA, USA) and transformed into Escherichia coli Top10 competent cells (Invitrogen), according to the manufacturer's instructions. The vectors from 10 positive colonies were purified using the PureYield Plasmid Mini Prep kit (Promega) and sequenced using the M13‐FP universal primer at Macrogen. The resulting chromatograms were analysed within Geneious v. 6.1.8 and aligned with the consensus sequences generated by the whole‐genome sequencing analysis using the ClustalW plugin within Geneious v. 6.1.8.
Phylogenetic analysis
EF‐1α, RPB1 and RPB2 were selected as phylogenetic loci from which to infer the phylogenetic relationships of the isolates used in this study. For each locus, the sequence reads from each isolate were mapped to a reference sequence from F. oxysporum originally identified by O'Donnell et al. (1998, 2013) (Table S1). Consensus sequences were generated for each gene and deposited at GenBank. The resulting sequences were employed to generate multiple sequence alignments using the ClustalW interface within Geneious v. 6.1.8 (Biomatters Ltd.). The multiple sequence alignments were manually inspected and gaps were removed prior to phylogenetic analysis. The concatenated and gap‐free alignment resulted in a sequence alignment of 4045 base pairs (EF‐1α, 621 bp; RPB1, 1607 bp; RPB2, 1817 bp).
The phylogenetic analysis of the concatenated EF‐1α/RPB1/RPB2 dataset was performed using MP, ML and BI methods. For each of the phylogenetic reconstruction methods, F. verticillioides was used as an outgroup in the analysis. The MP analysis was conducted within PAUP* v4.0α (Swofford, 2003), using a heuristic search and 1000 random sequence additions with tree bisection–reconnection on the partitioned dataset. Branch support was assessed using 1000 bootstraps. The ML analysis was performed using the software RAxML v8.1 (Stamatakis, 2014). The concatenated alignment was partitioned into the three genes and the ML tree was recovered from 1000 ML searches using the GTR + Γ substitution model for each of the partitioned gene sets. Branch support was determined from 1000 bootstrap replicates. The BI analysis was performed in MrBayes v3.2 (Huelsenbeck and Ronquist, 2001), in which the concatenated alignment was partitioned into the three genes and the GTR + G model of substitution was applied to each gene. Two independent analyses were conducted across four Markov chain Monte Carlo (MCMC) chains for 3 000 000 generations. The trees were sampled every 2000 generations and the first 750 000 trees were used as burn‐in. Convergence was inferred when the average split standard deviation reached <0.01 and when the log likelihood scores stabilized. The resulting trees were viewed and edited in the software FigTree (http://tree.bio.ed.ac.uk/software/figtree/).
Gene trees were also constructed for the SIX genes that were identified in the isolates screened in this study, including SIX1, SIX2, SIX6, SIX7, SIX8, SIX9, SIX10 and SIX13. Nucleotide sequence alignments were generated from the consensus sequences of the SIX genes identified in the isolates of Foc and other formae speciales screened in this study. In addition, SIX gene sequence data from the publically available genomes of F. oxysporum were included in the analyses to provide context. Nucleotide sequence alignments were performed using ClustalW within Geneious v. 6.1.8 and manually inspected. Gene trees were generated using the methods described above for the EF‐1α/RPB1/RPB2 dataset.
Phylogenetic hypothesis testing
A series of topological constraint tests was conducted to investigate the discordance between the SIX gene trees and the infraspecies phylogeny of F. oxysporum. The topological constraint analyses were only conducted with the datasets in which Foc‐SIX gene homologues were identified in isolates from both clades A and B of the infraspecies phylogeny. First, an ‘infraspecies constraint’ forced the taxa in the included SIX gene datasets to resemble the backbone of the understood infraspecies phylogeny of the F. oxysporum isolates included in this study. A ‘strict Foc‐SIX gene monophyly constraint’ forced the homologues of Foc‐SIX into a monophyletic clade, whereas the SIX homologues from other formae speciales were placed in a reciprocating sister polytomy. The ‘relaxed monophyly constraint’ was very similar to the strict monophyly constraint in that it forced most of the Foc‐SIX homologues and the SIX homologues from other formae speciales into monophyletic, reciprocating sister polytomies. However, unlike the strict monophyly constraint, the conflicting Foc‐SIX1i, For‐SIX9c, Foc‐SIX13d and Foc‐SIX13e homologues were allowed to freely sort across the constrained tree topology.
Each of the manually generated constraint trees was used as input in an ML analysis conducted in RAxML with 1000 bootstrap replicates. The resulting best‐site log likelihood scores from the ML analyses were compared for significant differences (P < 0.05) with those of the unconstrained trees using the Shimodaira–Hasegawa (SH) and Approximately Unbiased (AU) tests in CONSEL (http://www.sigmath.es.osaka-u.ac.jp/shimo-lab/prog/consel/; Shimodaira and Hasegawa, 2001).
Author Contributions
E.C., S.F.‐S., J.B. and E.A.B.A. planned and designed the research. E.C., S.F.‐S., M.Z., W.T.O.N., R.A.M. and L.T.T.T.‐N. performed the experiments. E.C. and S.F.‐S. analysed the data. E.C. and S.F.‐S. prepared the manuscript.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Table S1 The coding DNA sequences (CDSs) of the translation elongation factor (EF‐1α, 630 bp), RNA polymerase II subunit I (RPB1, 1607 bp) and subunit II (RPB2, 1847 bp) used to query the Fusarium oxysporum datasets analysed in this study for the purpose of constructing phylogenetic relationships.
Table S2 Accession details for the whole‐genome sequencing projects for the isolates of Fusarium oxysporum analysed in this study.
Table S3 Polymerase chain reaction (PCR) primers and thermocycling conditions for the amplification of Foc‐SIX gene homologues.
Acknowledgements
We would like to thank the Australian Banana Growers Council, Horticulture Australia (BA10020), for project funding. We thank the Australian Government, Australasian Plant Pathology Society and Australian Plant Biosecurity CRC for scholarship support. We thank Lucy Tran‐Nguyen, Cassie McMaster, Vu Tuan Nguyen, Dean Beasley, Yu Pei Tan and the Agricultural Research Service (NRRL) Culture Collection, US Department of Agriculture, USA for providing cultures.
References
- ABGC (Australian Banana Growers Council) (2015). Banana industry on alert after suspected case of TR4 in Tully. Available at: http://abgc.org.au/abgc-release-on-suspected-panama-tr4-case/ [accessed 4 March, 2015].
- Baayen, R.P. , O'Donnell, K. , Bonants, P.J.M. , Cigelnik, E. , Kroon, L.P.N.M. , Roebroeck, J.A. and Waalwijk, C. (2000) Gene genealogies and AFLP analyses in the Fusarium oxysporum complex identify monophyletic and nonmonophyletic formae speciales causing wilt and rot disease. Phytopathology, 90, 891–900. [DOI] [PubMed] [Google Scholar]
- Bentley, S. , Pegg, K.G. and Dale, J.L. (1995) Genetic variation among a world‐wide collection of isolates of Fusarium oxysporum f. sp. cubense analysed by RAPD‐PCR fingerprinting. Mycol. Res. 99, 1378–1384. [Google Scholar]
- Bentley, S. , Pegg, K.G. , Moore, N.Y. , Davis, R.D. and Buddenhagen, I.W. (1998) Genetic variation among vegetative compatibility groups of Fusarium oxysporum f. sp. cubense analyzed by DNA fingerprinting. Phytopathology, 88, 1283–1293. [DOI] [PubMed] [Google Scholar]
- Boehm, E.W.A. , Ploetz, R.C. and Kistler, H.C. (1994) Statistical analysis of electrophoretic karyotype variation among vegetative compatibility groups of Fusarium oxysporum f.sp cubense . Mol. Plant–Microbe Interact. 7, 1378–1384. [Google Scholar]
- Buddenhagen, I.W. (2009) Understanding strain diversity in Fusarium oxysporum f.sp. cubense and history of introduction of ‘tropical race 4’ to better manage banana production. Acta Hortic. 828, 193–204. [Google Scholar]
- Caten, C.E. and Jinks, J.L. (1966) Heterokaryosis: its significance in wild homothallic Ascomycetes and Fungi imperfecti. Trans. Br. Mycol. Soc. 49, 81–93. [Google Scholar]
- Chakrabarti, A. , Rep, M. , Wang, B. , Ashton, A. , Dodds, P. and Ellis, J. (2011) Variation in potential effector genes distinguishing Australian and non‐Australian isolates of the cotton wilt pathogen Fusarium oxysporum f.sp. vasinfectum . Plant Pathol. 60, 232–243. [Google Scholar]
- Covey, P.A. , Kuwitzky, B. , Hanson, M. and Webb, K.M. (2014) Multilocus analysis using putative fungal effectors to describe a population of Fusarium oxysporum from sugar beet. Phytopathology, 104, 886–896. [DOI] [PubMed] [Google Scholar]
- Forsyth, L.M. , Smith, L. and Aitken, E.A. (2006) Identification and characterization of non‐pathogenic Fusarium oxysporum capable of increasing and decreasing Fusarium wilt severity. Mycol. Res. 110, 929–935. [DOI] [PubMed] [Google Scholar]
- Fourie, G. , Steenkamp, E.T. , Gordon, T.R. and Viljoen, A. (2009) Evolutionary relationships among the Fusarium oxysporum f.sp. cubense vegetative compatibility groups. Appl. Environ. Microbiol. 75, 4770–4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser‐Smith, S. , Czislowski, E. , Meldrum, R.A. , Zander, M. , Balali, G.R. and Aitken, E.A.B. (2014) Sequence variation in the putative effector gene SIX8 facilitates molecular differentiation of Fusarium oxysporum f.sp. cubense . Plant Pathol. 63, 1044–1052. [Google Scholar]
- Garcia‐Bastidas, F. , Ordonez, N. , Konkol, J. , Al‐Qasim, M. , Naser, Z. , Abdelwali, M. , Salem, N. , Waalwijk, C. , Ploetz, R.C. and Kema, G.H.J. (2014) First report of Fusarium oxysporum f. sp. cubense tropical race 4 associated with Panama disease of banana outside Southeast Asia. Plant Dis. 98, 694. [DOI] [PubMed] [Google Scholar]
- Gawehns, F. , Houterman, P.M. , Ait Ichou, F. , Michielse, C.B. , Hijdra, M. , Cornelissen, B.J.C. , Rep, M. and Takken, F.L.W. (2014) The Fusarium oxysporum effector Six6 contributes to virulence and suppresses I‐2‐mediated cell death. Mol. Plant–Microbe Interact. 27, 336–348. [DOI] [PubMed] [Google Scholar]
- Gerlach, K.S. , Bentley, S. , Moore, N.Y. , Pegg, K.G. and Aitken, E.A.B. (2000) Characterisation of Australian isolates of Fusarium oxysporum f.sp. cubense by DNA fingerprinting. Aust. J. Agric. Res. 51, 945–953. [Google Scholar]
- Groenewald, S. , Van den Berg, N. , Marasas, W.F.O. and Viljoen, A. (2006) The application of high‐throughput AFLP's in assessing genetic diversity in Fusarium oxysporum f. sp. cubense . Mycol. Res. 110, 297–305. [DOI] [PubMed] [Google Scholar]
- Guo, L. , Han, L. , Yang, L. , Zeng, H. , Fan, D. , Zhu, Y. , Feng, Y. , Wang, G. , Peng, C. , Jiang, X. and Zhou, D. (2014) Genome and transcriptome analysis of the fungal pathogen Fusarium oxysporum f.sp. cubense causing banana vascular wilt disease. PLoS One, 9, e95543. doi: 10.1371/journal.pone.0095543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houterman, P.M. , Speijer, D. , Dekker, H.L. , De Koster, C.G. , Cornelissen, B.J.C. and Rep, M. (2007) The mixed xylem sap proteome of Fusarium oxysporum‐infected tomato plants. Mol. Plant Pathol. 8, 215–221. [DOI] [PubMed] [Google Scholar]
- Houterman, P.M. , Cornelissen, B.J.C. and Rep, M. (2008) Suppression of plant resistance gene‐based immunity by a fungal effector. PLoS Pathog. 4, e1000061. doi: 10.1371/journal.ppat.1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houterman, P.M. , Ma, L. , Van Ooijen, G. , De Vroomen, M.J. , Cornelissen, B.J.C. , Takken, F.L.W. and Rep, M. (2009) The effector protein Avr2 of the xylem‐colonizing fungus Fusarium oxysporum activates the tomato resistance protein I‐2 intracellularly. Plant J. 58, 970–978. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck, J.P. and Ronquist, F. (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics, 17, 754–755. [DOI] [PubMed] [Google Scholar]
- IPPC (International Plant Protection Convention) (2013). New banana disease found in Mozambique (Fusarium oxysporum f.sp. cubense tropical race 4). Available at: https://www.ippc.int/en/countries/mozambique/pestreports/2013/09/new-banana-disease-found-in-mozambique-fusarium-oxysporum-fspcubense-tropical-race-4/ [accessed 20 January, 2015].
- Jones, D.R. (2000) Diseases of Banana, Abaca and Enset. Wallingford, Oxon, UK: CABI Publishing.
- Jones, J.D. and Dangl, J.L. (2006) The plant immune system. Nature, 444, 323–329. [DOI] [PubMed] [Google Scholar]
- Katan, T. and Di Primo, P. (1999) Current status of vegetative compatibility groups in Fusarium oxysporum . Phytoparasitica, 27, 273–277. [Google Scholar]
- Kearse, M. , Moir, R. , Wilson, A. , Stones‐Havas, S. , Cheung, M. , Sturrock, S. , Buxton, S. , Cooper, A. , Markowitz, S. , Duran, C. and Thierer, T. (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28, 1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig, R.L. , Ploetz, R.C. and Kistler, H.C. (1997) Fusarium oxysporum f. sp. cubense consists of a small number of divergent and globally distributed clonal lineages. Phytopathology, 87, 915–923. [DOI] [PubMed] [Google Scholar]
- Laurence, M.H. , Summerell, B. and Liew, E.C.Y. (2015) Fusarium oxysporum f. sp. canariensis: evidence for horizontal gene transfer of putative pathogenicity genes. Plant Pathol. 64, 1068–1075. [Google Scholar]
- Lievens, B. , Houterman, P.M. and Rep, M. (2009) Effector gene screening allows unambiguous identification of Fusarium oxysporum f sp. lycopersici races and discrimination from other formae speciales . FEMS Microbiol. Lett. 300, 201–215. [DOI] [PubMed] [Google Scholar]
- Ma, L.J. , Van Der Does, H.C. , Borkovich, K.A. , Coleman, J.J. , Daboussi, M.J. , Di Pietro, A. , Dufresne, M. , Freitag, M. , Grabherr, M. , Henrissat, B. , and Houterman, P.M. (2010) Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium . Nature, 464, 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, L. , Cornelissen, B.J. and Takken, F.L. (2013) A nuclear localization for Avr2 from Fusarium oxysporum is required to activate the tomato resistance protein I‐2. Front. Plant Sci. 4, 94. doi: 10.3389/fpls.2013.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, L. , Houterman, P.M. , Gawehns, F. , Cao, L. , Sillo, F. , Richter, H. , Clavijo‐Ortiz, M.J. , Schmidt, S.M. , Boeren, S. , Vervoort, J. and Cornelissen, B.J. (2015) The AVR2–SIX5 gene pair is required to activate I‐2‐mediated immunity in tomato. New Phytol. 208, 507–518. [DOI] [PubMed] [Google Scholar]
- Meldrum, R.A. , Fraser‐Smith, S. , Tran‐Nguyen, L.T.T. , Daly, A.M. and Aitken, E.A.B. (2012) Presence of putative pathogenicity genes in isolates of Fusarium oxysporum f sp. cubense from Australia. Australas. Plant Pathol. 41, 551–557. [Google Scholar]
- Moore, N.Y. , Pegg, K.G. , Allen, R.N. and Irwin, J.A.G. (1993) Vegetative compatibility and distribution of Fusarium oxysporum f sp. cubense in Australia. Aust. J. Exp. Agric. 33, 797–802. [Google Scholar]
- Nucci, M. and Annaisie, E. (2007) Fusarium infections in immunocompromised patients. Clin. Microbiol. Rev. 20, 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell, K. , Kistler, H.C. , Cigelnik, E. and Ploetz, R.C. (1998) Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA, 95, 2044–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell, K. , Rooney, A.P. , Proctor, R.H. , Brown, R.H. , McCormich, S.P. , Ward, T.J. , Frandsen, R.J.N. , Lysoe, E. , Rehner, S.A. , Aoki, T. and Robert, V.A. (2013) Phylogenetic analyses of RPB1 and RPB2 support a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria. Fungal Genet. Biol. 52, 25–31. [DOI] [PubMed] [Google Scholar]
- O'Neill, W.T. , Pattison, A.B. , Daniells, J.W. , Hermanto, C. and Molina, A.B. (2011) Vegetative compatibility group analysis of Indonesian Fusarium oxysporum f. sp. cubense isolates. Acta Hortic. 897, 345–351. [Google Scholar]
- Ordonez, N. , Garcia‐Bastidas, F. , Laghari, H.B. , Akkary, M.Y. , Harfouche, E.N. , Al Awar, N.B. and Kema, G.H.J. (2016) First report of Fusarium oxysporum f. sp. cubense tropical race 4 causing Panama disease in Cavendish bananas in Pakistan and Lebanon. Plant Dis. 100, 209. [Google Scholar]
- Ortoneda, M. , Guarro, J. , Madrid, M.P. , Caracuel, Z. , Roncero, M.I. , Mayayo, E. and Di Pietro, A. (2004) Fusarium oxysporum as a multihost model for the genetic dissection of fungal virulence in plants and mammals. Infect. Immun. 72, 1760–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg, K.G. , Moore, N.Y. and Sorensen, S. (1994). Variability in populations of Fusarium oxysporum f.sp. cubense from the Asia/Pacific region In: The Improvement and Testing of Musa: A Global Partnership (Jones D.R., ed.), pp. 70–82. Montpellier: INIBAP. [Google Scholar]
- Ploetz, R.C. 1994. Fusarium wilt and IMTP Phase II In: The Improvement and Testing of Musa: A Global Partnership (Jones D.R., ed.), pp. 57–69. Montpellier: INIBAP. [Google Scholar]
- Ploetz, R.C. and Correll, J.C. (1988) Vegetative compatibility among races of Fusarium oxysporum f.sp. cubense . Plant Dis. 72, 325–328. [Google Scholar]
- Ploetz, R.C. , and Pegg, K.G. (2000). Fungal diseases of the root, corm and pseudostem. Fusarium wilt In: Diseases of Banana, Abaca and Enset (Jones D.R., ed,), pp. 143–159. Wallingford, Oxfordshire: CABI Publishing. [Google Scholar]
- Puhalla, J.E. (1985) Classification of strains of Fusarium oxysporum on the basis of vegetative compatibility. Can. J. Bot. 63, 179–183. [Google Scholar]
- Rep, M. , Van Der Does, H.C. , Meijer, M. , Van Wijk, R. , Houterman, P.M. , Dekker, H.L. , De Koster, C.G. and Cornelissen, B.J.C. (2004) A small, cysteine‐rich protein secreted by Fusarium oxysporum during colonization of xylem vessels is required for I‐3‐mediated resistance in tomato. Mol. Microbiol. 53, 1373–1383. [DOI] [PubMed] [Google Scholar]
- Rep, M. , Meijer, M. , Houterman, P.M. , Van Der Does, H.C. and Cornelissen, B.J.C. (2005) Fusarium oxysporum evades I‐3 mediated resistance without altering the matching avirulence gene. Mol. Plant–Microbe Interact. 18, 15–23. [DOI] [PubMed] [Google Scholar]
- Schmidt, S.M. , Houterman, P.M. , Schreiver, I. , Ma, L. , Amyotte, S. , Chellappan, B. , Boeren, S. , Takken, F.L. and Rep, M. (2013) MITEs in the promoters of effector genes allow prediction of novel virulence genes in Fusarium oxysporum . BMC Genomics, 14, 199. 10.1186/1471-2164-14-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimodaira, H. and Hasegawa, M. (2001) CONSEL: for assessing the confidence of phylogenetic tree selection. Bioinformatics, 17, 1246–1247. [DOI] [PubMed] [Google Scholar]
- Snyder, W.C. and Hansen, H.N. (1940) The species concept in Fusarium . Am. J. Bot. 27, 64–67. [Google Scholar]
- Stamatakis, A. (2014) RAxML Version 8: a tool for phylogenetic analysis and post‐analysis of large phylogenies. Bioinformatics, 30, 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover, R.H. (1990). Fusarium wilt of banana: some history and current status of the disease In: Fusarium Wilt of Banana (Ploetz R.C., ed.), pp. 1–7. St. Paul, MN: APS Press. [Google Scholar]
- Stover, R.H. and Buddenhagen, I.W. (1986) Banana breeding – polyploidy, disease resistance and productivity. Fruits, 41, 175–191. [Google Scholar]
- Stover, R.H. , and Simmonds, N.W. (1987). Bananas. Harlow, Essex: Longmans Scientific and Technica. [Google Scholar]
- Su, H.J. , Hwang, S.C. and Ko, W.H. (1986) Fusarial wilt of Cavendish bananas in Taiwan. Plant Dis. 70, 814–818. [Google Scholar]
- Swofford, D.L. (2003). PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Sunderland, MA: Sinauer Associates. [Google Scholar]
- Syed, R.N. , Lodhi, A.M. , Jiskani, M.M. , Rajput, K.I. , Khaskheli, M.A. , Khanzada, M.A. , Rajput, N.A. , Maitlo, S.A. and Rajput, A.Q. (2015) First report of Panama wilt disease of banana caused by Fusarium oxysporum f.sp. cubense in Pakistan. J. Plant Pathol. 97, 209–220. [Google Scholar]
- Takken, F. and Rep, M. (2010) The arms race between tomato and Fusarium oxysporum . Mol. Plant Pathol. 11, 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, A. , Vágány, V. , Jackson, A.C. , Harrison, R.J. , Rainoni, A. and Clarkson, J.P. (2016) Identification of pathogenicity‐related genes in Fusarium oxysporum f.sp. cepae . Mol. Plant Pathol. 17, 1032–1047. doi: 10.1111/mpp.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teetor‐Barsch, G.F. and Roberts, D.W. (1983) Entomogenous Fusarium species. Mycopathologia, 1, 3–16. [DOI] [PubMed] [Google Scholar]
- Thatcher, L.F. , Gardiner, D.M. , Kazan, K. and Manners, J.M. (2012) A highly conserved effector in Fusarium oxysporum is required for full virulence on Arabidopsis . Mol. Plant–Microbe Interact. 25, 180–190. [DOI] [PubMed] [Google Scholar]
- Van Dam, P. , Fokkens, L. , Schmidt, S.M. , Linmans, J.H.J. , Kistler, C. , Ma, L.‐J. and Rep, M. (2016) Effector profiles distinguish formae speciales of Fusarium oxysporum . Environ. Microbiol. 18, 336–348. doi: 10.1111/1462-2920.13445. [DOI] [PubMed] [Google Scholar]
- Waite, B.H. (1963) Wilt of Heliconia spp. caused by Fusarium oxysporum f.sp. cubense race 3. Trop. Agric. Res. 40, 299–305. [Google Scholar]
- Williams, A.H. , Sharma, M. , Thatcher, L.F. , Azam, A. , Hane, J.K. , Sperschneider, J. , Kidd, B.N. , Anderson, J.P. , Ghosh, R. , Garg, G. and Lichtenzveig, J. (2016) Comparative genomics and prediction of conditionally dispensable sequences in legume–infecting Fusarium oxysporum formae speciales facilitates identification of candidate effectors. BMC Genomics, 17, 191. doi: 10.1186/s12864-016-2486-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Table S1 The coding DNA sequences (CDSs) of the translation elongation factor (EF‐1α, 630 bp), RNA polymerase II subunit I (RPB1, 1607 bp) and subunit II (RPB2, 1847 bp) used to query the Fusarium oxysporum datasets analysed in this study for the purpose of constructing phylogenetic relationships.
Table S2 Accession details for the whole‐genome sequencing projects for the isolates of Fusarium oxysporum analysed in this study.
Table S3 Polymerase chain reaction (PCR) primers and thermocycling conditions for the amplification of Foc‐SIX gene homologues.


