Summary
Flagellin glycosylation plays a crucial role in flagellar assembly, motility and virulence in several pathogenic bacteria. However, little is known about the genetic determinants and biological functions of flagellin glycosylation in Xanthomonas oryzae pv. oryzae (Xoo), the causal pathogen of bacterial blight of rice. Here, the structure, regulation and functions of a ten‐gene cluster gigX (glycosylation island genes of Xoo), which was embedded in a flagellar regulon, were characterized. gigX1 to gigX10 encoded putative enzymes or proteins involved in glycan biosynthesis and transfer, including a nucleotide sugar transaminase, an acyl‐carrier protein (ACP), a 3‐oxoacyl‐ACP synthase, a 3‐oxoacyl‐ACP reductase, a dehydrogenase, an acetyltransferase, a ring hydroxylating dioxygenase, a hypothetical protein, a methyltransferanse and a glycosyltransferase, respectively. The gigX genes were co‐transcribed in an operon and up‐regulated by the upstream σ54 factor RpoN2 and transcriptional activator FleQ. In‐frame deletion of each gigX gene affected flagellin glycosylation modification, meaning that the unglycosylated flagellin of the mutants was smaller than the glycosylated flagellin of the wild‐type. No significant changes in flagellar filament and motility were observed in the ΔgigX mutants, among which only ΔgigX6 displayed increased swimming ability. Importantly, all mutants, except ΔgigX9, showed significantly increased virulence and bacterial growth in the susceptible rice cultivar IR24, and ΔgigX1 and ΔgigX10 showed enhanced type III secretion system (T3SS)‐related gene expression. Moreover, the glycosylated flagellin of the wild‐type induced higher H2O2 levels in rice leaves than did the unglycosylated flagellins of ΔgigX1 or ΔgigX10. Taken together, this study reveals that the gigX cluster determines flagellin glycosylation, and implicates the regulatory role of post‐translational modification with the glycosylation, acetylation and methylation of flagellin in the regulation of motility and virulence of Xoo.
Keywords: flagellar motility; flagellin glycosylation, gigX cluster; immune response; virulence; Xanthomonas oryzae pv. oryzae
Introduction
Xanthomonas oryzae pv. oryzae (Xoo) is one of the most important bacterial blight pathogens of rice, leading to 20%–50% yield losses, and has become an ideal model to study the molecular mechanisms of bacterial pathogenicity on monocot plants (Nino‐Liu et al., 2006). A range of virulence‐related factors, including extracellular polysaccharide (EPS) synthesis, biofilm formation, motility, extracellular cellulase, xylanase and adhesions, are produced by Xoo to facilitate its pathogenesis (Das et al., 2009; White and Yang, 2009). In addition to these virulence factors, like many other Gram‐negative bacteria, Xoo also possesses a type III secretion system (T3SS) to inject and deliver effectors into plant cells, some of which are critical determinants of pathogenic consequences during Xoo–rice interactions (Tsuge et al., 2006; White and Yang, 2009). In the Xoo T3SS, HrpG and HrpX are two major regulatory proteins that control the expression of structural genes, including hrcC, hrcU and hrcT, and the secretion of a number of effector proteins (Song and Yang, 2010; White and Yang, 2009). Therefore, it is of both scientific and economic significance to elucidate the regulatory mechanisms underlying how the virulence factors are expressed in Xoo.
A flagellar gene cluster containing over 60 contiguous genes in the genome of PXO99A has been identified previously; the genes putatively encode proteins with various functions, including structural components, protein export apparatus, regulatory factors, chemotaxis, GGDEF/EAL domain proteins, which are involved in the cyclic‐di‐guanosine monophosphate (cyclic‐di‐GMP) signal transduction pathway, and proteins for post‐translational modification (Tian et al., 2015). Within this gene cluster, a genomic island (GI) containing 10 genes was annotated to be responsible for flagellin glycosylation (Salzberg et al., 2008).
Flagellin glycosylation is a complicated biochemical process of post‐translational modification, which is generally encoded by a number of clustered genes in several pathogenic bacteria (Chaban et al., 2006; Taguchi et al., 2006). For example, a GI containing 14 genes that determined flagellin glycosylation was first identified in Pseudomonas aeruginosa strain PAK, which encoded the enzymes and proteins involved in flagellin glycosylation, such as glycosyltransferase, acyl‐carrier protein (ACP) and acetyltransferase (Arora et al., 2001). The inactivation of genes in GI abolished flagellin glycosylation, indicating the involvement of GI in flagellin glycosylation (Arora et al., 2001). In Pseudomonas syringae pv. tabaci 6605, a viosamine gene cluster contains six dTDP‐mVio biosynthetic genes, including vioA for dTDP‐viosamine aminotransferase, vioM for methyltransferase, vioS for 3‐oxoacyl‐ACP synthase, vioB for dTDP‐viosamine acetyltransferase, vioR for 3‐oxoacyl‐ACP reductase and acp for ACP, and one mVio glycosyltransferase gene vioT (Nguyen et al., 2009). dTDP‐mVio is a substrate for VioT and is required for the incorporation of mVio into the flagellin glycan, demonstrating a viosamine metabolic pathway involved in flagellin glycosylation in P. syringae (Nguyen et al., 2009; Yamamoto et al., 2011). All of the genes displayed the corresponding homology in GI of P. aeruginosa strain PAK (Yamamoto et al., 2011). In addition, three clustered genes, fgt1, fgt2 and orf3, were found upstream of the flagellin gene fliC in P. syringae pv. tabaci 6605 (Taguchi et al., 2006). Flagellins from the Δfgt1 and Δfgt2 mutants were not glycosylated and partially glycosylated, respectively, indicating that both genes encode glycosyltransferases, which are required for flagellin glycosylation (Taguchi et al., 2006; Takeuchi, 2008). In addition to P. syringae, flagellin glycosylation has also been reported in the rice virulent strain K1 of Acidovorax avenae, in which four genes, fgt, fmt, fcs and fst, encoding putative glycosyltransferase, methyltransferase, carbamoylphosphate synthase and sugar transaminase, respectively, have been identified to regulate the glycosylation of flagellin (Hirai et al., 2014). In Xanthomonas campestris pv. campestris (Xcc), there is a five gene‐containing GI, including a glycosyltransferase gene fgt, a nucleotide sugar transaminase gene vioA and three hypothetical protein genes (Ichinose et al., 2013). Moreover, flagellin glycosylation of Burkholderia glumae strain Pg‐10, Dickeya dadantii strain 92‐31, Pantoea ananatis strain AZ200124, Pseudomonas cichorii strain KN52, Pectobacterium carotovorum ssp. carotovorum strain EC1 and Xcc strain XcA has been demonstrated, suggesting that flagellin glycosylation is ubiquitous in a broad range of plant‐pathogenic bacteria (Ichinose et al., 2013).
Although flagellin glycosylation has been revealed in further pathogenic bacteria, in many cases, its biological function remains to be elucidated (Logan, 2006; Merino and Tomas, 2014). In general, flagellin glycosylation affects filament assembly, stability and flagellar motility (Asakura et al., 2010; Guerry et al., 2006; Taguchi et al., 2006, 2008, 2010), virulence (Khodai‐Kalaki et al., 2015; Lithgow et al., 2014), host specificity, recognition and innate immune evasion (Logan, 2006; Nothaft and Szymanski, 2010). Flagellin glycosylation has been implicated in the regulation of bacterial virulence and interactions with the host in several pathogenic bacteria (Arora et al., 2005; Khodai‐Kalaki et al., 2015; Taguchi et al., 2006). For instance, the P. aeruginosa mutant with unglycosylated flagellin was significantly attenuated in virulence to humans (Arora et al., 2005). In Burkholderia pseudomallei and Burkholderia thailandensis, it has been suggested that flagellar glycosylation could be a mechanism used to modulate virulence, given the difference in glycan composition and virulence (Scott et al., 2011). Pseudomonas syringae pv. tabaci flagellin glycosylation was also required for the full virulence of the pathogen by evasion of the host plant surveillance system (Taguchi et al., 2006, 2008, 2009, 2010). Burkholderia cenocepacia flagellin glycosylation contributed to the evasion of the human innate immune response, but not to innate immune recognition in Arabidopsis thaliana (Hanuszkiewicz et al., 2014; Khodai‐Kalaki et al., 2015). In most cases, flagellin glycosylation plays a positive role in bacterial virulence expression and helps the bacterial pathogen to evade host plant innate immunity.
Although flagellin glycosylation plays a crucial role in flagellar assembly, motility and virulence in several pathogenic bacteria, little is known about the genetic determinants and biological functions of flagellin glycosylation in Xoo. A ten‐gene cluster named gigX (glycosylation island genes of Xoo), embedded in a flagellar regulon, was revealed in the Xoo genome (Salzberg et al., 2008). Mutation of PXO_00987, encoding a putative acetyltransferase, affected flagellin glycosylation (Li et al., 2015). In this study, the structure, regulation and functions of the gigX cluster were further characterized through genetic and biochemical analysis. The gigX genes were homologous with the GI genes found in P. aeruginosa, which encode enzymes and proteins involved in the glycosylation process. Co‐transcription of gigX genes was up‐regulated by the transcriptional regulator FleQ and alternative sigma factor RpoN2. The deletion of each of these genes resulted in the complete abolishment of flagellin glycosylation, whereas flagellar assembly and motility were not affected in most mutants. Importantly, bacterial virulence on rice cultivar IR24 was significantly increased in most mutants with unglycosylated flagellin. This study provides further insights into the post‐translational modification of flagellin, and implicates its role in the regulation of motility and virulence in plant‐pathogenic bacteria.
Results
gigX genes encode enzymes/proteins involved in the glycosylation process
In the genome of the wild‐type strain PXO99A, there are two identical copies of the flagellar gene cluster consisting of more than 60 genes encoding proteins with various functions, including structural components, protein export apparatus, regulatory factors and post‐translational modification enzymes (Tian et al., 2015). A putative glycosylation island that was embedded in the flagellar regulon consists of 10 genes, named gigX1–gigX10 (gene annotation #: PXO_00983–PXO_00992 and PXO_06174–PXO_06183). The genetic organization of this GI locus showed that the complete nucleotide sequence of the gigX genes was 11 555 bp in length and located between the upstream transcriptional regulator encoding gene fleQ and the downstream basebody encoding gene fliE (Fig. 1A). gigX1–gigX10 were annotated to encode a nucleotide sugar transaminase, ACP, 3‐oxoacyl‐ACP synthase, 3‐oxoacyl‐ACP reductase, acetyltransferase, hydroxylating dioxygenase, ring hydroxylating dioxygenase, hypothetical protein, methyltransferase and glycosyltransferase, respectively (Table 1). In addition, bioinformatics analysis showed that some of these genes were highly homologous to the GI genes in P. aeruginosa strain PAK and P. syringae pv. tabaci strain 6605, which were required for the protein glycosylation process (Table 1). Thus, these findings indicate that the gigX genes might be involved in the protein glycosylation process, such as glycan biosynthesis and transfer.
Figure 1.
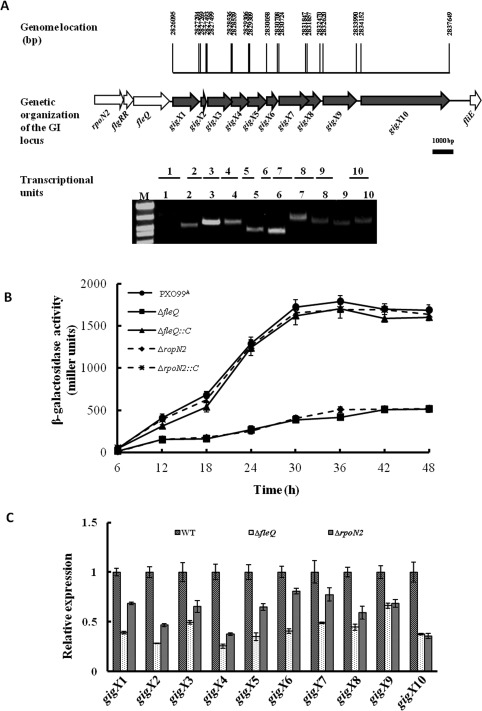
Transcriptional regulation of gigX genes in Xanthomonas oryzae pv. oryzae (Xoo) strains. (A) Schematic diagram of the gigX cluster in the genome of Xoo strain PXO99A. Open arrows indicate the length, location and orientation of the open reading frames (ORFs). The short lines below the arrows indicate the location and length of the reverse transcription polymerase chain reaction (RT‐PCR) products. The bottom element shows the RT‐PCR analysis of RNA isolated from PXO99A. RT‐dependent amplification of DNA fragments suggested that the genomic island (GI) genes were transcribed in one operon. (B) β‐Galactosidase activity assay of the gigX promoter. The activity of the gigX promoter in PXO99A, ΔfleQ, ΔfleQ‐C, ΔrpoN2 and ΔrpoN2‐C was detected. The experiments were repeated three times, independently. (C) Quantitative real‐time polymerase chain reaction (qRT‐PCR) analysis of gigX gene transcription in ΔfleQ and ΔrpoN2. The data represent the relative expression level of gigX in ΔfleQ and ΔrpoN2. The error bar represents standard deviations from three biological repeats. WT, wild‐type.
Table 1.
Similarities of glycosylation island genes in Xanthomonas oryzae pv. oryzae PXO99A, Pseudomonas aeruginosa PAK and Pseudomonas syringae pv. tabaci 6605.
| PXO99A | PAK | 6605 | ||||||
|---|---|---|---|---|---|---|---|---|
| Gene | Cluster A | Cluster B | Size of product (amino acid) | Annotation | Cluster | % identity (amino acid) | Cluster | % identity (amino acid) |
| gigX1 | PXO_06174 | PXO_00992 | 369 | Nucleotide sugar transaminase | orfA | 55 | vioA | 64 |
| gigX2 | PXO_06175 | PXO_00991 | 74 | Acyl‐carrier protein (ACP) | orfB | 72 | acp | 20 |
| gigX3 | PXO_06176 | PXO_00990 | 346 | 3‐Oxoacyl‐ACP synthase | orfC | 81 | vioS | 31 |
| gigX4 | PXO_06177 | PXO_00989 | 257 | 3‐Oxoacyl‐ACP reductase | orfD | 38 | vioR | 38 |
| gigX5 | PXO_06178 | PXO_00988 | 250 | Dehydrogenase | – | – | – | – |
| gigX6 | PXO_06179 | PXO_00987 | 216 | Acetyltransferase | orfE | 44 | vioB | 32 |
| gigX7 | PXO_06180 | PXO_00986 | 381 | Ring hydroxylating dioxygenase α‐subunit | orfF | 35 | – | – |
| gigX8 | PXO_06181 | PXO_00985 | 207 | Hypothetical protein | orfG | 25 | – | – |
| gigX9 | PXO_06182 | PXO_00984 | 507 | Methyltransferase | orfL | 11 | vioM | 6 |
| gigX10 | PXO_06183 | PXO_00983 | 1183 | Glycosyltransferase | orfN | 45 | vioT | 39 |
Co‐transcribed gigX genes are regulated by σ54 factor RpoN2 and transcriptional activator FleQ
Alternative sigma factor σ54 RpoN and transcriptional activator FleQ have been shown to regulate flagellar gene expression in several bacteria (Prouty et al., 2001). Our earlier work has shown that the expression of flagellar regulatory and structural genes is significantly down‐regulated in the rpoN2 and fleQ deletion mutants (Tian et al., 2015). To assess the regulation by RpoN2 and FleQ of gigX genes, we first demonstrated that all gigX genes were co‐transcribed in one operon belonging to one transcriptional unit using reverse transcription polymerase chain reaction (RT‐PCR) analysis, and this transcriptional unit did not include fleQ (Fig. 1A). Next, bioinformatics analysis of the first gigX gene (gigX1) promoter sequence identified the NNGGN10 GCNN consensus sequence upstream of the transcription initiation site of gigX1, which is recognized by RpoN2‐containing RNA polymerase, with the putative binding site centred at −12 and −24 nucleotides with a 10‐nucleotide spacing between these consensus sequences. Thus, the gigX1 promoter was fused with the lacZ gene and the resulting gigX1‐p::lacZ was analysed for activity by evaluation of the β‐galactosidase assay in ΔrpoN2, ΔfleQ and relevant complementation strains. The β‐galactosidase assay showed that the transcriptional activity of the gigX1 promoter decreased significantly in both ΔrpoN2 and ΔfleQ, which could be restored to near wild‐type level in the relevant complementation strains (Fig. 1B). Furthermore, quantitative real‐time polymerase chain reaction (qRT‐PCR) assay demonstrated that all gigX gene transcripts were remarkably reduced in ΔrpoN2 and ΔfleQ, and restored in part in the relevant complementation strains (Fig. 1C). These results suggest that the regulation of co‐transcribed gigX genes is positively controlled by RpoN2 and FleQ in Xoo.
Deletion in each gigX gene affects flagellin glycosylation
To demonstrate the functions of the gigX cluster in flagellin glycosylation, in‐frame deletion was performed for each of the gigX genes, and the flagellin proteins from the wild‐type and mutant strains were extracted, purified and assayed for their glycosylation, as described in Experimental Procedures. Sodium dodecylsulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) and Coomassie brilliant blue staining analysis showed that the sizes of the flagellins in the mutants of each gigX, except gigX9 encoding a methyltransferase, were smaller than those of the wild‐type PXO99A, which was further confirmed by Western blot analysis (Fig. 2A). To determine that such a change in flagellin size was a result of protein glycosylation, a glycoprotein staining experiment was performed using a Pierce glycoprotein staining kit. As predicted, the flagellins from the wild‐type and ΔgigX9 were glycosylated, whereas those from the other mutants were unglycosylated (Fig. 2A), indicating that deletion in the gigX genes abolished flagellin glycosylation. Matrix‐assisted laser desorption/ionization‐time of flight (MALDI‐TOF) mass spectrometry (MS) analysis showed that the unglycosylated flagellin of ΔgigX10 had a molecular mass about 3 kDa less than the glycosylated flagellin of the wild‐type (Fig. 2B). In addition, although the wild‐type and ΔgigX9 flagellins showed the heterogeneous glycoform, the value of each peak tended to be small in ΔgigX9 (Fig. 2B), indicating that GigX9 might function as a methyltransferase potentially involved in the methylation of flagellin and/or glycan. These findings reveal that all gigX genes determine the post‐translational modification of flagellin in Xoo.
Figure 2.
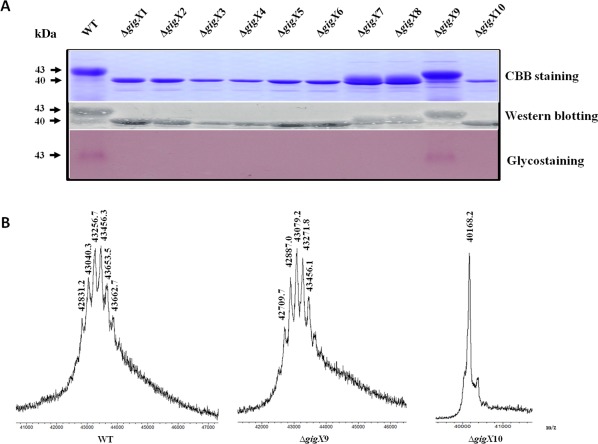
Flagellin glycosylation in Xanthomonas oryzae pv. oryzae strains. (A) Detection of the purified flagellin proteins using Coomassie brilliant blue (CBB) staining, Western blotting and glycosyl moiety staining. (B) Matrix‐assisted laser desorption/ionization‐time of flight (MALDI‐TOF) mass spectrometry (MS) analysis of flagellin proteins. The flagellins from the wild‐type and ΔgigX9 displayed similar MS peaks with extensive heterogeneity in glycoform distribution. The molecular mass of flagellin from other gigX gene mutants was about 3 kDa less than that of the wild‐type. WT, wild‐type.
No changes in flagellar filament and motility occur in ΔgigX, except ΔgigX6
To investigate the roles of flagellin glycosylation in the flagellar motility of Xoo, the flagellar filament was visualized using transmission electron microscopy, and swimming and swarming zones were measured after bacterial growth at 28 °C for 2 and 4 days on 0.25% and 0.5% agar‐containing plates, respectively. The flagellum of the ΔgigX mutants was found to be similar to that of the wild‐type (Fig. 3A), suggesting that the deletion of gigX genes had no effect on flagellar assembly. Moreover, there was no significant difference in the average diameters of the swimming zone between the wild‐type and mutants, except for ΔgigX6, which produced a larger swimming zone than the wild‐type (Fig. 3B), indicating the inhibitory role of GigX6 in swimming motility. Furthermore, all ΔgigX mutants showed similar diameters of the swarming zone to the wild‐type (Fig. 3C). Overall, these observations demonstrate that GigX might not be required for filament production and flagellar motility, with the exception of GigX6, which suppresses swimming motility in Xoo.
Figure 3.
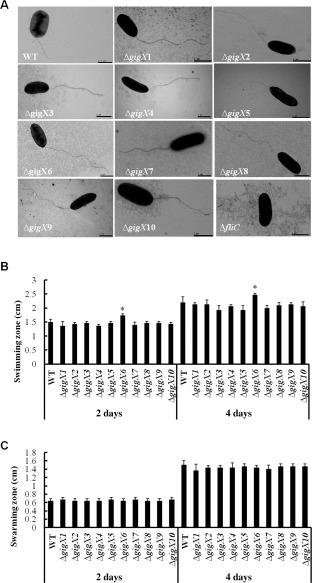
Flagellar filament, swimming and swarming motility of Xanthomonas oryzae pv. oryzae strains. (A) Visualization of flagellar filament using scanning electron microscopy. (B) Measurement of swimming zones after bacterial growth for 2 and 4 days on semisolid plates (0.03% peptone, 0.03% yeast extract and 0.25% agar) at 28 °C. (C) Measurement of swarming zones after bacterial growth for 2 and 4 days on SWM plates (0.5% peptone, 0.3% yeast extract and 0.5% agar) at 28 °C. Error bars indicate standard deviations. The experiments were repeated three times, independently. WT, wild‐type.
Deletion in each gigX, except gigX9, enhances virulence and bacterial growth in rice
To further determine the contribution of gigX to the virulence of Xoo in rice, the pathogenicity of ΔgigX1–ΔgigX10 and their complementation strains on susceptible rice cultivar IR24 was tested by leaf‐clipping inoculation. The disease symptoms were recorded by photography and the lesion lengths were measured at 14 days post‐inoculation. Compared with the wild‐type PXO99A, all ΔgigX mutants, except ΔgigX9, showed significantly enhanced ability to cause disease on rice leaves, including more severe bacterial blight symptoms (Fig. 4A) and longer lesions (Fig. 4B). The expression of each gigX in trans in the relevant mutants restored the disease phenotypes to near wild‐type levels. Similarly, all ΔgigX mutants, except ΔgigX9, displayed a significant increase in bacterial growth in rice leaf tissues in comparison with the wild‐type (Fig. 4C). ΔgigX9 showed the same virulent phenotype and in planta growth in rice as the wild‐type. Moreover, assays for the bacterial growth rate of the wild‐type and mutants demonstrated no significant differences when grown in nutrient‐rich medium M210 (Fig. S1, see Supporting Information), excluding the possibility that the enhanced virulence of the mutants was caused by a change in growth in vitro. Accordingly, these data indicate that the gigX genes, except gigX9, are involved in the virulence and in planta growth of Xoo.
Figure 4.
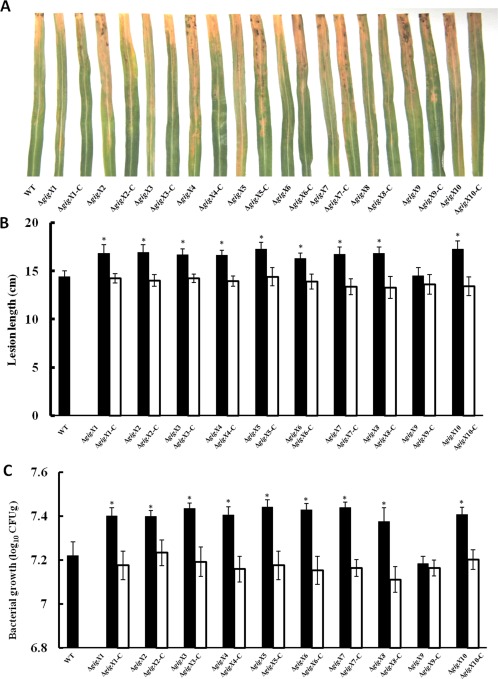
Virulence on susceptible rice cultivar IR24 of Xanthomonas oryzae pv. oryzae (Xoo) strains. (A) Disease symptoms were recorded at 14 days after inoculation by photography. Xoo strains were inoculated by leaf clipping after bacterial growth had reached an optical density at 600 nm (OD600) of 0.8. (B) Disease lesion lengths of rice were measured at 14 days after inoculation. At least 10 leaves were assayed for each Xoo strain in each independent experiment. (C) Bacterial population was detected at 14 days after inoculation. Three leaves were mixed as one sample for each strain. The experiments were repeated three times, independently. Error bars represent standard deviations, and asterisk indicates P < 0.05 (Student's t‐test). CFU, colony‐forming unit; WT, wild‐type.
ΔgigX1 and ΔgigX10 show enhanced T3SS‐related gene expression
T3SS contributes to virulence through the injection of effector proteins into the cytosol of host cells in pathogenic bacteria (Jones and Dangl, 2006). Based on the observation of changes in virulence of the ΔgigX mutants, it was speculated that the functions of their T3SS might be affected. To test this hypothesis, the transcripts of two T3SS regulatory genes, hrpG and hrpX, one effector harpin gene, hpa1, and three structural genes, hrcC, hrcU and hrcT, were compared among the wild‐type strain, ΔgigX1 and ΔgigX10 mutants, and relevant complementation strains. The expression of the T3SS‐related genes was induced in XOM2, a nutrient‐scarce minimal medium which has been considered to mimic growth conditions in planta (Furutani et al., 2003), followed by the extraction of total RNA from the wild‐type and mutants. The transcript levels of each gene in the mutants relative to that in the wild‐type, which was designated as unity, were calculated. No significant change in the mRNA level of hrpG was found in either mutant; however, the transcripts of hrpX, hrp1, hrcC, hrcU and hrcT were significantly increased in ΔgigX1 and ΔgigX10 relative to the wild‐type (Fig. 5), suggesting that either gigX1 or gigX10 has a negative effect on hrp gene expression. Complementation of ΔgigX1 and ΔgigX10 by in trans expression of gigX1 and gigX10, respectively, restored hrp gene expression to near wild‐type levels (Fig. 5). These observations demonstrate that GigX1 and GigX10 negatively regulate T3SS expression at the transcriptional level.
Figure 5.
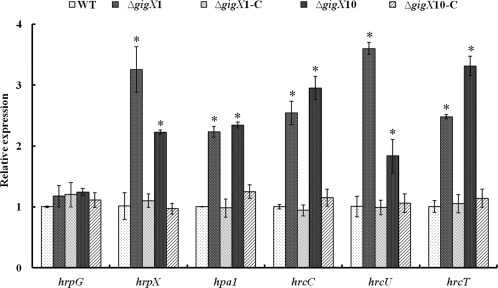
Transcriptional levels of type III secretion system (T3SS)‐related genes in Xanthomonas oryzae pv. oryzae strains. The relative expression of T3SS‐related genes was detected by qRT‐PCR in the wild‐type, ΔgigX1 and ΔgigX10, and the fold changes of each gene were calculated using the 2–ΔΔCt method. Error bars represent standard deviations from three biological repeats, and asterisk indicates P < 0.05 by Student's t‐test.
No changes in EPS production and biofilm formation are found in ΔgigX, except ΔgigX6
As the ΔgigX mutants displayed increased virulence in rice, two important virulence‐related factors of Xoo, EPS production and biofilm formation, were investigated in this study. No significant differences in EPS secretion and biofilm formation were found between the wild‐type and ΔgigX mutants, except ΔgigX6, which displayed significantly less EPS production and biofilm formation (Figs S2 and S3, see Supporting Information). These results suggest that GigX6, but not other GigXs, regulate EPS secretion and biofilm formation in Xoo.
Higher H2O2 level is induced by glycosylated flagellin in rice leaves
To assess the role of flagellin glycosylation in pathogen‐associated molecular pattern (PAMP)‐triggered immunity (PTI) in rice, the production of H2O2, one of the reactive oxygen species (ROS), in glycosylated or unglycosylated flagellin‐treated rice leaves was measured via luminal chemiluminescence assay, with flg22, one of the typical PAMPs, used as a positive control. The wild‐type glycosylated flagellin induced higher H2O2 levels in rice leaves than the ΔgigX1 or ΔgigX10 unglycosylated flagellins and flg22 (Fig. 6). These results suggest that Xoo glycosylated flagellin might elicit a stronger PTI in rice.
Figure 6.
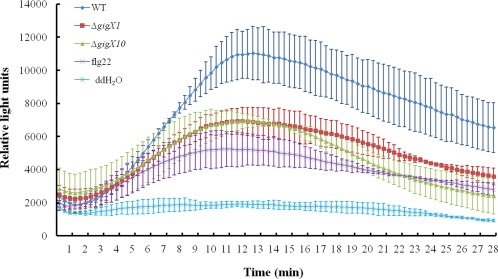
H2O2 generation in flagellin‐treated rice leaves. Leaf discs (4 mm in diameter) of rice susceptible cultivar IR24 were treated with 100 nm flagellin from the wild‐type, ΔgigX1 or ΔgigX10; 100 nm flg22 was used as a positive control. The experiments were repeated at least three times, independently. The error bars represent standard deviations.
Discussion
To elucidate the genetic determinants and biological roles of flagellin glycosylation in Xoo, we identified the structure of gigX, a 10‐gene‐containing GI responsible for flagellin glycosylation, characterized its regulation of expression by the σ54 factor RpoN2 and transcriptional activator FleQ, and analysed the functions in flagellar motility and virulence through bioinformatics and genetic analysis. We demonstrated that the RpoN2/FleQ‐directed gigX cluster plays a crucial role in glycan biosynthesis, nine genes of which (except the gigX9‐encoding methyltransferase) were required for flagellin glycosylation. To date, only a few flagellin glycosylation pathways have been elucidated in pathogenic bacteria, including the Wbp and Vio pathways of P. aeruginosa and P. syringae, respectively (Larkin and Imperiali, 2009; Larkin et al., 2010; Yamamoto et al., 2011). To our immediate knowledge, this is the first report to identify a tandem of genes that were co‐transcribed under the direction of RpoN2 and FleQ, and to determine the flagellin glycosylation process in Xoo.
Our demonstration of the flagellin glycosylation pathway (designed as the GigX pathway) encoded by the gigX cluster in Xoo is supported by the following experimental evidence. First, the structure and predicted function of the gigX genes were quite similar to those of P. aeruginosa and P. syringae (Table 1). In P. aeruginosa, the Wbp pathway was encoded by 14 GI genes encoding the sugar biosynthetic and transfer enzymes, including glycosyltransferase, acetyltransferase, methyltransferase, 3‐oxoacyl‐ACP synthase and reductase, and nucleotide sugar transaminase (Arora et al., 2001). In P. syringae, two GIs were identified, in which two genes from the smaller GI encoded glycosyltransferase, whereas seven genes from the larger GI showed high homology with the P. aeruginosa GI genes and encoded the Vio pathway (Taguchi et al., 2006; Yamamoto et al., 2011). Second, the genetic analysis of gigX through gene deletion mutagenesis and glycosylation detection of flagellins extracted from the wild‐type and mutant strains confirmed that all gigX genes were necessary for the post‐translational modification of flagellin with glycosylation, acetylation and methylation (Fig. 2), suggesting that flagellin glycosylation requires glycosyltransferase, acetyltransferase, 3‐oxoacyl‐ACP synthase and reductase, and nucleotide sugar transaminase, etc. The biochemical activities and modes of action of each of the gigX‐encoded enzymes and proteins in the process of glycan biosynthesis and transfer will be further investigated. The identification of flagellin glycans is also required for comprehensive elucidation of the GigX pathway in Xoo.
The effect of flagellin glycosylation on swimming or swarming motility varied in P. syringae and P. aeruginosa in each non‐identical experimental system (Arora et al., 2005; Taguchi et al., 2006, 2008). For example, flagellin glycosylation affected surface swarming on 0.5% semisolid agar plates and swimming motility in a highly viscous liquid medium, but did not affect surface swimming on 0.3% agar plates in P. syringae, whereas flagellin glycosylation did not influence surface swimming on 0.325% agar plates in P. aeruginosa. We observed that swimming motility on 0.25% agar plates was not affected in most ΔgigX mutants, except ΔgigX6 (Fig. 3B), and swarming motility on 0.5% agar plates was not altered in all gigX mutants (Fig. 3C), indicating no effects of flagellin glycosylation on flagellar motility in Xoo. Combined with other investigations, this study reveals the functional differences of flagellin glycosylation in the regulation of bacterial motility.
Glycosylated flagellins have been identified in other pathogenic bacteria; however, the knowledge of their biological role in the regulation of pathogen virulence and host innate immune responses remains limited. In general, flagellin glycosylation positively promotes bacterial virulence expression and host innate susceptibility. In this study, however, we observed that all ΔgigX mutants, except ΔgigX9, showed significantly enhanced virulence and bacterial growth in planta (Fig. 4), and gigX1 and gigX10, the first and last genes of the gigX cluster, encoding nucleotide sugar transaminase and glycosyltransferase, respectively, both displayed a negative effect on T3SS‐related hrp gene expression (Fig. 5). In addition, in trans expression of each gene in the relevant mutants restored the virulent phenotype to near wild‐type levels (Figs 4 and 5). Therefore, this is the first study to report that gigX‐mediated glycosylation modification of flagellin negatively affects virulence in rice. Although more detailed mechanisms underlying such a novel finding need to be further explored, the possible explanations are proposed as follows. First, glycosylated flagellin may be recognized by the host surveillance system to elicit a strong PTI, thus resulting in attenuated virulence and in planta growth of Xoo in rice. This is evidenced, in part, by our current observation that glycosylated flagellin elicits higher H2O2 levels than unglycosylated flagellin in leaves of rice IR24 (Fig. 6). This is in contrast with the current view that flagellin glycosylation functions to evade the plant surveillance system (Hanuszkiewicz et al., 2014; Khodai‐Kalaki et al., 2015; Taguchi et al., 2006, 2008, 2009, 2010). Second, the bacterial glycan composition itself, other than flagellin, which is generated through the GigX pathway, may act directly as a PAMP for PTI induction in rice. Finally, other components, such as O‐antigens, lipopolysaccharide and pili, which are targeted and modified by the GigX system, are novel virulence factors which may affect or facilitate bacterial pathogenesis in rice. Further experiments are required to confirm the plausible hypotheses and to identify the differences in response to glycosylated or unglycosylated flagellins in different rice cultivars. Future investigations should address two important questions. Is this case a ubiquitous phenomenon or rather an exception with regard to the role of flagellin glycosylation? Why has Xoo evolved such a complicated GigX system to glycosylate flagellins, which is always effectively recognized to elicit a strong PTI in rice?
gigX6, encoding a putative acetyltransferase GigX6, is required for flagellin glycosylation (Li et al., 2015), EPS production and biofilm formation (Table 1, Figs 2A, S2 and S3), and GigX6 inhibition affects swimming motility (Fig. 3B). These observations indicate that acetyltransferase might play a critical role in the regulation of flagellin glycosylation, flagellar motility, EPS production and biofilm formation. In P. syringae, acetyltransferase VioB is essential for flagellin glycosylation, and vioB mutation strongly impairs swarming motility and weakly reduces swimming motility (Nguyen et al., 2009; Yamamoto et al., 2011). In Xanthomonas spp., acetyltransferases are specifically involved in the EPS biogenesis pathway (Katzen et al., 1998; Kim et al., 2009). Further studies are needed to reveal the molecular mechanism by which GigX6‐mediated acetylation affects motility, EPS production and biofilm formation in Xoo.
gigX9 encodes a putative methyltransferase (Table 1). The position of the flagellin band in SDS‐PAGE analysis is not drastically changed in ΔgigX9 compared with the wild‐type, but the value of each mass spectral peak of flagellin glycans tends to be smaller than those of the wild‐type (Fig. 2B), suggesting that the flagellin glycan may be unmethylated. Similar results have also been reported in the methyltransferase gene vioM in the flagellin GI of P. syringae pv. tabaci 6605 (Yamamoto et al., 2011). However, the relationship of flagellin methylation to bacterial virulence remains to be elucidated. We found that gigX9‐mediated methylation is not required for glycosylated flagellin to affect virulence on rice in Xoo. This finding helps us to better understand the regulatory role of flagellin methylation in bacterial virulence.
Xcc, the crucifer black rot pathogen taxonomically close to Xoo, has a five gene‐containing GI of flagellin including the glycosyltransferase gene fgt, and Δfgt shows significantly reduced motility and virulence on cabbage (Ichinose et al., 2013). Sequence alignment analysis reveals that Xoo GigX10 and Xcc Fgt show 46% amino acid identity (Fig. S4, see Supporting Information). The molecular mass of Xoo flagellin glycan is 1 kDa larger than that of Xcc. In addition, GigX10 and Fgt displayed different roles in virulence on host plants. Thus, significant differences in terms of glycan composition and regulation of the virulence phenotype of flagellins might exist between the two bacterial pathogens.
Overall, the current study provides novel insights into the post‐translational modification with glycosylation, acetylation and methylation of flagellin, and implicates its regulatory role in motility, EPS production, biofilm formation and virulence in Xoo.
Experimental Procedures
Bacterial strains, plasmids and growth conditions
The bacterial strains and plasmids used in this study are listed in Table 2. Xoo strains were grown in M210 liquid medium or in peptone sucrose agar (PSA) solid medium at 28 °C, and Escherichia coli strains were maintained in Luria–Bertani medium at 37 °C. The antibiotics ampicillin (Ap), gentamicin (Gm), streptomycin (Sp) and kanamycin (Km) were used at 100, 50, 50 and 50 µg/mL, respectively.
Table 2.
Bacterial strains and plasmids used in this study.
| Strain or plasmid | Relevant characteristics* | Reference or source |
|---|---|---|
| Escherichia coli | ||
| DH5α | supE44 ΔlacU169(Φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi‐1 relA1 | Hanahan (1983) |
| Xanthomonas oryzae pv. oryzae | ||
| PXO99A | Wild‐type strain, Philippine race 6 | Laboratory collection |
| ΔgigX1 | gigX1 gene deletion mutant derived from PXO99A | This study |
| ΔgigX2 | gigX2 gene deletion mutant derived from PXO99A | This study |
| ΔgigX3 | gigX3 gene deletion mutant derived from PXO99A | This study |
| ΔgigX4 | gigX4 gene deletion mutant derived from PXO99A | This study |
| ΔgigX5 | gigX5 gene deletion mutant derived from PXO99A | This study |
| ΔgigX6 | gigX6 gene deletion mutant derived from PXO99A | Our laboratory |
| ΔgigX7 | gigX7 gene deletion mutant derived from PXO99A | This study |
| ΔgigX8 | gigX8 gene deletion mutant derived from PXO99A | This study |
| ΔgigX9 | gigX9 gene deletion mutant derived from PXO99A | This study |
| ΔgigX10 | gigX10 gene deletion mutant derived from PXO99A | This study |
| ΔgigX1‐C | ΔgigX1 containing plasmid pBBR‐gigX1, Apr | This study |
| ΔgigX2‐C | ΔgigX2 containing plasmid pBBR‐gigX2, Apr | This study |
| ΔgigX3‐C | ΔgigX3 containing plasmid pBBR‐gigX3, Apr | This study |
| ΔgigX4‐C | ΔgigX4 containing plasmid pBBR‐gigX4, Apr | This study |
| ΔgigX5‐C | ΔgigX5 containing plasmid pBBR‐gigX5, Apr | This study |
| ΔgigX6‐C | ΔgigX6 containing plasmid pBBR‐gigX6, Apr | Our laboratory |
| ΔgigX7‐C | ΔgigX7 containing plasmid pBBR‐gigX7, Apr | This study |
| ΔgigX8‐C | ΔgigX8 containing plasmid pBBR‐gigX8, Apr | This study |
| ΔgigX9‐C | ΔgigX9 containing plasmid pBBR‐gigX9, Apr | This study |
| ΔgigX10‐C | ΔgigX10 containing plasmid pBBR‐gigX10, Apr | This study |
| ΔfleQ | fleQ gene deletion mutant derived from PXO99A, Gmr | Our laboratory |
| ΔfleQ‐C | ΔfleQ containing plasmid pHM1‐fleQ, Spr | Our laboratory |
| ΔrpoN2 | rpoN2 gene deletion mutant derived from PXO99A, Gmr | Our laboratory |
| ΔrpoN2‐C | ΔrpoN2 containing plasmid pHM1‐rpoN2, Spr | Our laboratory |
| ΔfliC | fliC gene deletion mutant derived from PXO99A, Gmr | Our laboratory |
| Plasmid | ||
| pKMS1 | Suicidal vector carrying sacB gene for non‐marker mutagenesis, Kmr | Li et al. (2011) |
| pKM‐gigX1 | pKMS1 derivative carrying a gigX1 mutation, Kmr | This study |
| pKM‐gigX2 | pKMS1 derivative carrying a gigX2 mutation, Kmr | This study |
| pKM‐gigX3 | pKMS1 derivative carrying a gigX3 mutation, Kmr | This study |
| pKM‐gigX4 | pKMS1 derivative carrying a gigX4 mutation, Kmr | This study |
| pKM‐gigX5 | pKMS1 derivative carrying a gigX5 mutation, Kmr | This study |
| pKM‐gigX7 | pKMS1 derivative carrying a gigX7 mutation, Kmr | This study |
| pKM‐gigX8 | pKMS1 derivative carrying a gigX8 mutation, Kmr | This study |
| pK18mobsacB | Suicidal vector carrying sacB gene for mutagenesis, Kmr | Schafer et al. (1994) |
| pK18‐gigX6 | pK18mobSacB derivative carrying a gigX6 mutation, Kmr, Gmr | Our laboratory |
| pK18‐gigX9 | pK18mobSacB derivative carrying a gigX9 mutation, Kmr, Gmr | This study |
| pK18‐gigX10 | pK18mobSacB derivative carrying a gigX10 mutation, Kmr, Gmr | This study |
| pBBR1MCS‐4 | Broad‐host‐range expression vector, Apr | Kovach et al. (1995) |
| pBBR‐gigX1 | pBBR1MCS‐4 carrying the full length of gigX1, Apr | This study |
| pBBR‐gigX2 | pBBR1MCS‐4 carrying the full length of gigX2, Apr | This study |
| pBBR‐gigX3 | pBBR1MCS‐4 carrying the full length of gigX3, Apr | This study |
| pBBR‐gigX4 | pBBR1MCS‐4 carrying the full length of gigX4, Apr | This study |
| pBBR‐gigX5 | pBBR1MCS‐4 carrying the full length of gigX5, Apr | This study |
| pBBR‐gigX6 | pBBR1MCS‐4 carrying the full length of gigX6, Apr | Our laboratory |
| pBBR‐gigX7 | pBBR1MCS‐4 carrying the full length of gigX7, Apr | This study |
| pBBR‐gigX8 | pBBR1MCS‐4 carrying the full length of gigX8, Apr | This study |
| pBBR‐gigX9 | pBBR1MCS‐4 carrying the full length of gigX9, Apr | This study |
| pBBR‐gigX10 | pBBR1MCS‐4 carrying the full length of gigX10, Apr | This study |
| pHM1 | Broad‐host‐range expression vector, Spr | Hopkins et al. (1992) |
| pHM1‐P | pHM1 carrying the gigX promoter and a promoterless lacZ gene, Spr | This study |
*Apr, Kmr, Spr and Gmr indicate resistance to ampicillin, kanamycin, streptomycin and gentamicin, respectively.
Bioinformatics analysis of the gigX cluster
The gene location, size of products and annotation of the gigX genes in Xoo wild‐type strain PXO99A, and the homologous sequences of gigX genes in P. aeruginosa strain PAK and P. syringae pv. tabaci strain 6605, were obtained from the National Center for Biotechnology Information (NCBI) website. Relevant sequence alignment was performed using DNAMAN software (Lynnon Biosoft, San Ramon, CA, USA).
Generation of gigX1‐p::lacZ and assay for β‐galactosidase activity
A DNA fragment (350 bp) containing the predicted −12/–24 region of the gigX promoter was amplified from PXO99A genomic DNA using specific primers (gigXpF/R), and ligated into the modified pHM1 vector containing a promoterless lacZ gene. The recombinant pHM1‐P was introduced into PXO99A, ΔrpoN2, ΔfleQ and their complementary strains. The strains transformed with pHM1‐P were selected by resistance to streptomycin. These strains were grown as described previously to measure the β‐galactosidase activity in cellular extracts using the β‐Galactosidase Enzyme Assay System (Promega, Madison, WI, USA) (Zhan et al., 2008). Assays were performed with three biological replicates. The primer sequences are listed in Table S1 (see Supporting Information).
RNA isolation and qRT‐PCR analysis
Bacterial strains were cultured in M210 medium until an optical density at 600 nm (OD600) of 0.6 was attained. Cells were then collected by centrifugation at 12 000 g. To induce the expression of T3SS‐related genes, Xoo strains were subcultured in XOM2 medium at 28 °C for 12 h and harvested by centrifugation at 12 000 g for 5 min (Yang et al., 2015). Total RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and treated with DNase, and the cDNA fragments were synthesized using Superscript III reverse transcriptase (Invitrogen) with random primers. The gyrB gene was used as a reference gene for qRT‐PCR assays. The qRT‐PCR analysis was performed as described previously (Yu et al., 2014) with primers designed using Primer Premier 5.0 software (PREMIER Biosoft, Palo Alto, CA, USA) (Table S1). Three biological replicates and triplicate PCR were tested for every sample.
Construction of gene deletion mutants and complementation strains
The gene deletion mutants of gigX derived from PXO99A were generated by homologous recombination using the suicide vector pKMS1, as reported previously (Li et al., 2015, 2011). In brief, the left and right arms of the gene were amplified by PCR from Xoo genomic DNA with the relevant F/R primers (Table S1). These fragments were ligated into suicide vector pKMS1, resulting in plasmid pKM‐Y (Y represents the name of the gigX gene). Then, pKM‐Y was introduced into PXO99A by electroporation. The transformant was first selected on NAN medium (1% tryptone, 0.1% yeast extract, 0.3% peptone and 1.5% agar) containing Km, followed by continuous transfer culture in NBN liquid medium (1% sucrose) five times. The candidates were screened on NAS medium (10% sucrose). The gene deletion mutant that could grow on NAS, but was sensitive to Km, was validated by PCR analysis. To generate the complementary strain, the coding region and promoter of the gene were amplified with the relevant F/R primers (Table S1) and inserted into vector pBBR1MCS‐4, and the recombinant plasmids were electroporated into the relevant mutants for complementation analysis. Finally, these complementary strains were further confirmed by PCR analysis.
Extraction and purification of flagellin proteins
Purification of flagellin proteins was performed as described previously with some modifications (Taguchi et al., 2006). Briefly, Xoo wild‐type and mutants were inoculated in M210 for 48 h at 28 °C, and collected by centrifugation at 12 000 g for 5 min. The cells were resuspended in 1/3 vol MMMF medium (50 mm potassium phosphate buffer, 7.6 mm (NH4)2SO4, 1.7 mm MgCl2, 1.7 mm NaCl, 10 mm mannitol and 10 mm fructose, pH 5.7) for 24 h at 23 °C. Then, the cells were harvested by centrifugation and resuspended in 50 mm sodium phosphate medium (pH 7.0), and violently shaken for 3 min. After centrifugation at 7000 g for 10 min, the supernatant was filtered through a 0.45‐µm pore size filter and centrifuged at 100 000 g for 1 h; pellets of purified flagellin were obtained and suspended in sterilized double‐distilled water (ddH2O) at a final concentration of 100 µg/mL.
Western blotting and glycosylation detection of flagellin
Purified flagellin proteins (10 µL) from Xoo wild‐type and mutant strains were separated by SDS‐PAGE at 100 V for 1.5 h, and electroblotted onto a poly(vinylidene difluoride) (PVDF) membrane at 20 V for 1 h. Then, the flagellin proteins were detected by an anti‐flagellin first antibody and goat anti‐rabbit second antibody (Beijing Protein Innovation, Beijing, China) conjugated with horseradish peroxidase (Tiangen Biotech, Beijing, China). For glycoprotein analysis, the purified flagellin proteins (10 µL) were detected by a GelCode glycoprotein detection kit (Pierce Biotechnology, Rockford, IL, USA) after SDS‐PAGE.
MS analysis of flagellin
Purified flagellin proteins from Xoo strains were subjected to MS as described previously (Taguchi et al., 2006). Flagellins were dialysed in water with 0.1% (v/v) trifluoroacetic acid (TFA), and added to an equal volume of mixture containing a saturated solution of sinapinic acid in 33% acetonitrile–water with 0.1% TFA (v/v). The mixtures were deposited on a stainless steel target plate, and the mass spectra of flagellins were analysed by a Biflex III MALDI‐TOF mass spectrometer (Bruker, Ibaraki, Japan) in a linear, positive mode with a mass accuracy of 0.2%.
Electron microscopy visualization of flagellum
Xoo strains were grown on PSA medium at 28 ºC for 48 h, and suspended in ddH2O. Then, the suspension was deposited onto grids coated with Formvar (Standard Technology, Ormond Beach, FL, USA), stained with 2% uranyl acetate for 30 s and dried for 10 min at room temperature. The flagella were observed using a transmission electron microscope (H‐7500, Hitachi, Tokyo, Japan).
Flagellar motility assay
Xoo strains were grown in M210 medium until OD600 of 0.8 was reached. The cells were harvested by centrifugation at 12 000 g for 5 min and resuspended in ddH2O. For swimming and swarming motility assays, 2 µL of bacterial suspension were inoculated onto semisolid medium plates (0.03% peptone, 0.03% yeast extract and 0.25% agar) and SWM plates (0.5% peptone, 0.3% yeast extract and 0.5% agar), respectively, and incubated at 28 °C for 2 and 4 days; the diameters of the bacterial swimming and swarming zones were measured. The experiments were repeated three times, independently.
Pathogenicity assay
The Xoo wild‐type and mutant strains were grown in M210 medium at 28 °C for 48 h, collected by centrifugation at 7 000 g for 15 min and resuspended in ddH2O (OD600 = 0.8). The virulence of these strains was detected on susceptible rice (Oryza sativa L. ssp. indica IR24) by leaf clipping, and the lesion lengths of 10 leaves were recorded at 14 days after inoculation for every strain. The population of bacteria on inoculated leaves was also detected. Three inoculated leaves were ground in ddH2O with a mortar and grinding rod, and the mixture was diluted to optical concentration, and spread onto PSA plates. The colonies of bacteria were counted after incubation at 28 °C for 72 h. The experiments were repeated three times, independently.
In vitro growth rate measurement
Xoo strains were grown in M210 medium until OD600 = 0.8. For bacterial growth assay, 200 µL of bacteria were inoculated in 100 mL of M210 liquid medium and cultured at 28 °C at 200 rpm. Then, the growth curves of these strains were obtained by testing OD600 every 6 h. The experiments were repeated three times, independently.
Exopolysaccharide production assay
To estimate exopolysaccharide production, bacterial strains were grown in M210 medium at 28 °C at OD600 = 2.5, and the supernatants were collected by centrifugation at 7000 g for 15 min. Two volumes of ethanol were added to the supernatants, and the mixture was held at −20 °C for 2 h. EPS was obtained by centrifugation at 12 000 g for 10 min and dried at 55 °C for 48 h. Finally, the dry weights were recorded as an estimate of EPS (Yang et al., 2014).
Biofilm formation assay
The biofilm formation assay was performed as described previously (Yang et al., 2014). Briefly, bacteria were grown in M210 at 200 rpm to OD600 = 0.5, and then 5 mL were transferred to tubes and standing inoculated at 28 °C for 72 h. Bacterial pellicles were stained with 0.1% crystal violet for 15 min at room temperature. The tubes were rinsed gently with water and photographed. Finally, the biofilm was dissolved with ethanol, and the absorbance at 490 nm was recorded. The experiments were repeated three times, independently.
Assay for H2O2 production in flagellin‐treated rice leaves
H2O2 production assay was performed as described previously (Ding et al., 2012). In brief, leaf discs (4 mm in diameter) were excised from 6‐week‐old rice plants (Oryza sativa L. ssp. indica IR24). After incubation in ddH2O for 12 h, three leaf discs were treated with a mixture containing 100 nm of flagellin, 100 µL luminal solution (Thermo Scientific, Rockford, IL, USA), 10 nm horseradish peroxidase (Sigma‐Aldrich, St Louis, MO, USA) and 200 µL ddH2O. The flagellins were extracted and purified from the wild‐type, ΔgigX1 and ΔgigX10 as described above; 100 nm of flg22, a 22‐amino‐acid containing peptide (QRLSTGSRINSAKDDAAGLQIA) that was artificially synthesized (BGI, Shenzhen, China), was used as a positive control. Luminescence was recorded every 20 s for 28 min on a Glomas 20/20 luminometer (Promega). The experiments were repeated at least three times, independently.
Statistical analysis
Disease lesion length, bacterial population measurement and relative gene expression were presented as means ± standard deviation. Student's t‐test was performed with statistical significance set at the 0.05 confidence level.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 Bacterial growth in vitro of Xanthomonas oryzae pv. oryzae strains. Bacterial growth in M210 medium was determined by measuring the optical density at 600 nm (OD600) at 6‐h intervals. Experiments were performed with three biological replicates.
Fig. S2 Exopolysaccharide production of Xanthomonas oryzae pv. oryzae (Xoo) strains. Extracellular polysaccharide (EPS) secretion in Xoo strains was detected by examination of the colony and quantification of EPS production. Values represent the average of three independent experiments, and the error bars indicate the standard deviations.
Fig. S3 Biofilm formation of Xanthomonas oryzae pv. oryzae (Xoo) strains. Biofilm formation in Xoo strains was quantified. The values represent the average of three independent experiments, and the error bars indicate the standard deviations.
Fig. S4 Sequence alignment analysis of glycosyltransferase from Xanthomonas oryzae pv. oryzae PXO99A and Xanthomonas campestris pv. campestris ATCC33913. The sequences were downloaded from the National Center for Biotechnology Information (NCBI) website, and sequence alignment was performed using DNAMAN software. The shading indicates identical amino acids.
Table S1 Primers used in this study
Acknowledgements
This work was supported by grants from the National Basic Research Program, China (2011CB100700) and National High‐Technology Research Program, China (2012AA101504) for C.H.
References
- Arora, S.K. , Bangera, M. , Lory, S. and Ramphal, R. (2001) A genomic island in Pseudomonas aeruginosa carries the determinants of flagellin glycosylation. Proc. Natl. Acad. Sci. USA, 98, 9342–9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora, S.K. , Neely, A.N. , Blair, B. , Lory, S. and Ramphal, R. (2005) Role of motility and flagellin glycosylation in the pathogenesis of Pseudomonas aeruginosa burn wound infections. Infect. Immun. 73, 4395–4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura, H. , Churin, Y. , Bauer, B. , Boettcher, J.P. , Bartfeld, S. , Hashii, N. , Kawasaki, N. , Mollenkopf, H.J. , Jungblut, P.R. , Brinkmann, V. and Meyer, T.F. (2010) Helicobacter pylori HP0518 affects flagellin glycosylation to alter bacterial motility. Mol. Microbiol. 78, 1130–1144. [DOI] [PubMed] [Google Scholar]
- Chaban, B. , Voisin, S. , Kelly, J. , Logan, S.M. and Jarrell, K.F. (2006) Identification of genes involved in the biosynthesis and attachment of Methanococcus voltae N‐linked glycans: insight into N‐linked glycosylation pathways in Archaea. Mol. Microbiol. 61, 259–268. [DOI] [PubMed] [Google Scholar]
- Das, A. , Rangaraj, N. and Sonti, R.V. (2009) Multiple adhesin‐like functions of Xanthomonas oryzae pv. oryzae are involved in promoting leaf attachment, entry, and virulence on rice. Mol. Plant–Microbe Interact, 22, 73–85. [DOI] [PubMed] [Google Scholar]
- Ding, B. , Bellizzi Mdel, R. , Ning, Y. , Meyers, B.C. and Wang, G.L. (2012) HDT701, a histone H4 deacetylase, negatively regulates plant innate immunity by modulating histone H4 acetylation of defense‐related genes in rice. Plant Cell, 24, 3783–3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furutani, A. , Tsuge, S. , Oku, T. , Tsuno, K. , Inoue, Y. , Ochiai, H. , Kaku, H. and Kubo, Y. (2003) Hpa1 secretion via type III secretion system in Xanthomonas oryzae pv. oryzae . J. Gen. Plant Pathol. 69, 271–275. [Google Scholar]
- Guerry, P. , Ewing, C.P. , Schirm, M. , Lorenzo, M. , Kelly, J. , Pattarini, D. , Majam, G. , Thibault, P. and Logan, S. (2006) Changes in flagellin glycosylation affect Campylobacter autoagglutination and virulence. Mol. Microbiol. 60, 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan, D. (1983) Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166, 557–580. [DOI] [PubMed] [Google Scholar]
- Hanuszkiewicz, A. , Pittock, P. , Humphries, F. , Moll, H. , Rosales, A.R. , Molinaro, A. , Moynagh, P.N. , Lajoie, G.A. and Valvano, M.A. (2014) Identification of the flagellin glycosylation system in Burkholderia cenocepacia and the contribution of glycosylated flagellin to evasion of human innate immune responses. J. Biol. Chem. 289, 19 231–19 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai, H. , Takai, R. , Kondo, M. , Furukawa, T. , Hishiki, T. , Takayama, S. and Che, F.S. (2014) Glycan moiety of flagellin in Acidovorax avenae K1 prevents the recognition by rice that causes the induction of immune responses. Plant Signal. Behav. 9, e972782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins, C.M. , White, F.F. , Choi, S.H. , Guo, A. and Leach, J.E. (1992) Identification of a family of avirulence genes from Xanthomonas oryzae pv. oryzae . Mol. Plant–Microbe Interact, 5, 451–459. [DOI] [PubMed] [Google Scholar]
- Ichinose, Y. , Taguchi, F. , Yamamoto, M. , Ohnishi‐Kameyama, M. , Atsumi, T. , Iwaki, M. , Manabe, H. , Kumagai, M. , Nguyen, Q.T. , Nguyen, C.L. , Inagaki, Y. , Ono, H. , Chiku, K. , Ishii, T. and Yoshida, M. (2013) Flagellin glycosylation is ubiquitous in a broad range of phytopathogenic bacteria. J. Gen. Plant Pathol. 79, 359–365. [Google Scholar]
- Jones, J.D. and Dangl, J.L. (2006) The plant immune system. Nature, 444, 323–329. [DOI] [PubMed] [Google Scholar]
- Katzen, F. , Ferreiro, D.U. , Oddo, C.G. , Ielmini, M.V. , Becker, A. , Puhler, A. and Ielpi, L. (1998) Xanthomonas campestris pv. campestris gum mutants: effects on xanthan biosynthesis and plant virulence. J. Bacteriol. 180, 1607–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodai‐Kalaki, M. , Andrade, A. , Fathy Mohamed, Y. and Valvano, M.A. (2015) Burkholderia cenocepacia lipopolysaccharide modification and flagellin glycosylation affect virulence but not innate immune recognition in plants. mBio, 6, e00679‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S.Y. , Kim, J.G. , Lee, B.M. and Cho, J.Y. (2009) Mutational analysis of the gum gene cluster required for xanthan biosynthesis in Xanthomonas oryzae pv oryzae . Biotechnol. Lett. 31, 265–270. [DOI] [PubMed] [Google Scholar]
- Kovach, M.E. , Elzer, P.H. , Hill, D.S. , Robertson, G.T. , Farris, M.A. , Roop, R.M., 2nd . and Peterson, K.M. (1995) Four new derivatives of the broad‐host‐range cloning vector pBBR1MCS, carrying different antibiotic‐resistance cassettes. Gene, 166, 175–176. [DOI] [PubMed] [Google Scholar]
- Larkin, A. and Imperiali, B. (2009) Biosynthesis of UDP‐GlcNAc(3NAc)A by WbpB, WbpE, and WbpD: enzymes in the Wbp pathway responsible for O‐antigen assembly in Pseudomonas aeruginosa PAO1. Biochemistry, 48, 5446–5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin, A. , Olivier, N.B. and Imperiali, B. (2010) Structural analysis of WbpE from Pseudomonas aeruginosa PAO1: a nucleotide sugar aminotransferase involved in O‐antigen assembly. Biochemistry, 49, 7227–7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithgow, K.V. , Scott, N.E. , Iwashkiw, J.A. , Thomson, E.L. , Foster, L.J. , Feldman, M.F. and Dennis, J.J. (2014) A general protein O‐glycosylation system within the Burkholderia cepacia complex is involved in motility and virulence. Mol. Microbiol. 92, 116–137. [DOI] [PubMed] [Google Scholar]
- Li, H.Y. , Yu, C. , Chen, H.M. , Tian, F. and He, C.Y. (2015) PXO_00987, a putative acetyltransferase, is required for flagellin glycosylation, and regulates flagellar motility, exopolysaccharide production, and biofilm formation in Xanthomonas oryzae pv. oryzae . Microb. Pathog. 85, 50–57. [DOI] [PubMed] [Google Scholar]
- Li, Y.R. , Zou, H.S. , Che, Y.Z. , Cui, Y.P. , Guo, W. , Zou, L.F. , Chatterjee, S. , Biddle, E.M. , Yang, C.H. and Chen, G.Y. (2011) A novel regulatory role of HrpD6 in regulating hrp‐hrc‐hpa genes in Xanthomonas oryzae pv. oryzicola . Mol. Plant–Microbe Interact, 24, 1086–1101. [DOI] [PubMed] [Google Scholar]
- Logan, S.M. (2006) Flagellar glycosylation – a new component of the motility repertoire? Microbiology, 152, 1249–1262. [DOI] [PubMed] [Google Scholar]
- Merino, S. and Tomas, J.M. (2014) Gram‐negative flagella glycosylation. Int. J. Mol. Sci. 15, 2840–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, L.C. , Yamamoto, M. , Ohnishi‐Kameyama, M. , Andi, S. , Taguchi, F. , Iwaki, M. , Yoshida, M. , Ishii, T. , Konishi, T. , Tsunemi, K. and Ichinose, Y. (2009) Genetic analysis of genes involved in synthesis of modified 4‐amino‐4,6‐dideoxyglucose in flagellin of Pseudomonas syringae pv. tabaci . Mol. Genet. Genomics, 282, 595–605. [DOI] [PubMed] [Google Scholar]
- Nino‐Liu, D.O. , Ronald, P.C. and Bogdanove, A.J. (2006) Xanthomonas oryzae pathovars: model pathogens of a model crop. Mol. Plant Pathol. 7, 303–324. [DOI] [PubMed] [Google Scholar]
- Nothaft, H. and Szymanski, C.M. (2010) Protein glycosylation in bacteria: sweeter than ever. Nat. Rev. Microbiol. 8, 765–778. [DOI] [PubMed] [Google Scholar]
- Prouty, M.G. , Correa, N.E. and Klose, K.E. (2001) The novel sigma54‐ and sigma28‐dependent flagellar gene transcription hierarchy of Vibrio cholerae . Mol. Microbiol. 39, 1595–1609. [DOI] [PubMed] [Google Scholar]
- Salzberg, S.L. , Sommer, D.D. , Schatz, M.C. , Phillippy, A.M. , Rabinowicz, P.D. , Tsuge, S. , Furutani, A. , Ochiai, H. , Delcher, A.L. , Kelley, D. , Madupu, R. , Puiu, D. , Radune, D. , Shumway, M. , Trapnell, C. , Aparna, G. , Jha, G. , Pandey, A. , Patil, P.B. , Ishihara, H. , Meyer, D.F. , Szurek, B. , Verdier, V. , Koebnik, R. , Dow, J.M. , Ryan, R.P. , Hirata, H. , Tsuyumu, S. , Won Lee, S. , Seo, Y.S. , Sriariyanum, M. , Ronald, P.C. , Sonti, R.V. , Van Sluys, M.A. , Leach, J.E. , White, F.F. and Bogdanove, A.J. (2008) Genome sequence and rapid evolution of the rice pathogen Xanthomonas oryzae pv. oryzae PXO99A . BMC Genomics, 9, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, A. , Tauch, A. , Jager, W. , Kalinowski, J. , Thierbach, G. and Puhler, A. (1994) Small mobilizable multi‐purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum . Gene, 145, 69–73. [DOI] [PubMed] [Google Scholar]
- Scott, A.E. , Twine, S.M. , Fulton, K.M. , Titball, R.W. , Essex‐Lopresti, A.E. , Atkins, T.P. and Prior, J.L. (2011) Flagellar glycosylation in Burkholderia pseudomallei and Burkholderia thailandensis . J. Bacteriol. 193, 3577–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, C.F. and Yang, B. (2010) Mutagenesis of 18 type III effectors reveals virulence function of XopZ(PXO99) in Xanthomonas oryzae pv. oryzae . Mol. Plant–Microbe Interact, 23, 893–902. [DOI] [PubMed] [Google Scholar]
- Taguchi, F. , Takeuchi, K. , Katoh, E. , Murata, K. , Suzuki, T. , Marutani, M. , Kawasaki, T. , Eguchi, M. , Katoh, S. , Kaku, H. , Yasuda, C. , Inagaki, Y. , Toyoda, K. , Shiraishi, T. and Ichinose, Y. (2006) Identification of glycosylation genes and glycosylated amino acids of flagellin in Pseudomonas syringae pv. tabaci . Cell. Microbiol. 8, 923–938. [DOI] [PubMed] [Google Scholar]
- Taguchi, F. , Shibata, S. , Suzuki, T. , Ogawa, Y. , Aizawa, S. , Takeuchi, K. and Ichinose, Y. (2008) Effects of glycosylation on swimming ability and flagellar polymorphic transformation in Pseudomonas syringae pv. tabaci 6605. J. Bacteriol. 190, 764–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi, F. , Suzuki, T. , Takeuchi, K. , Inagaki, Y. , Toyoda, K. , Shiraishi, T. and Ichinose, Y. (2009) Glycosylation of flagellin from Pseudomonas syringae pv. tabaci 6605 contributes to evasion of host tobacco plant surveillance system. Physiol. Mol. Plant Pathol. 74, 11–17. [Google Scholar]
- Taguchi, F. , Yamamoto, M. , Ohnishi‐Kameyama, M. , Iwaki, M. , Yoshida, M. , Ishii, T. , Konishi, T. and Ichinose, Y. (2010) Defects in flagellin glycosylation affect the virulence of Pseudomonas syringae pv. tabaci 6605. Microbiology, 156, 72–80. [DOI] [PubMed] [Google Scholar]
- Takeuchi, K. (2008) Studies on flagellin glycosylation and pathogenicity of Pseudomonas syringae . J. Gen. Plant Pathol. 74, 461–462. [Google Scholar]
- Tian, F. , Yu, C. , Li, H.Y. , Wu, X.L. , Li, B. , Chen, H.M. , Wu, M.S. and He, C.Y. (2015) Alternative sigma factor RpoN2 is required for flagellar motility and full virulence of Xanthomonas oryzae pv. oryzae . Microbiol. Res. 170, 177–183. [DOI] [PubMed] [Google Scholar]
- Tsuge, S. , Nakayama, T. , Terashima, S. , Ochiai, H. , Furutani, A. , Oku, T. , Tsuno, K. , Kubo, Y. and Kaku, H. (2006) Gene involved in transcriptional activation of the hrp regulatory gene hrpG in Xanthomonas oryzae pv. oryzae . J. Bacteriol. 188, 4158–4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, F.F. and Yang, B. (2009) Host and pathogen factors controlling the rice–Xanthomonas oryzae interaction. Plant Physiol. 150, 1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, M. , Ohnishi‐Kameyama, M. , Nguyen, C.L. , Taguchi, F. , Chiku, K. , Ishii, T. , Ono, H. , Yoshida, M. and Ichinose, Y. (2011) Identification of genes involved in the glycosylation of modified viosamine of flagellins in Pseudomonas syringae by mass spectrometry. Genes, 2, 788–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, F. , Tian, F. , Li, X.T. , Fan, S.S. , Chen, H.M. , Wu, M.S. , Yang, C.H. and He, C.Y. (2014) The degenerate EAL‐GGDEF domain protein Filp functions as a cyclic di‐GMP receptor and specifically interacts with the PilZ‐domain protein PXO_02715 to regulate virulence in Xanthomonas oryzae pv. oryzae . Mol. Plant–Microbe Interact, 27, 578–589. [DOI] [PubMed] [Google Scholar]
- Yang, F.H. , Tian, F. , Chen, H.M. , Hutchins, W. , Yang, C.H. and He, C.Y. (2015) The Xanthomonas oryzae pv. oryzae PilZ domain proteins function differentially in cyclic di‐GMP binding and regulation of virulence and motility. Appl. Environ. Microbiol. 81, 4358–4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, C. , Chen, H.M. , Tian, F. , Leach, J.E. and He, C.Y. (2014) Differentially‐expressed genes in rice infected by Xanthomonas oryzae pv. oryzae relative to a flagellin‐deficient mutant reveal potential functions of flagellin in host–pathogen interactions. Rice, 7, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan, L.J. , Han, Y.P. , Yang, L. , Geng, J. , Li, Y.L. , Gao, H. , Guo, Z.B. , Fan, W. , Li, G. , Zhang, L.F. , Qin, C. , Zhou, D.S. and Yang, R.F. (2008) The cyclic AMP receptor protein, CRP, is required for both virulence and expression of the minimal CRP regulon in Yersinia pestis biovar microtus. Infect. Immun. 76, 5028–5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 Bacterial growth in vitro of Xanthomonas oryzae pv. oryzae strains. Bacterial growth in M210 medium was determined by measuring the optical density at 600 nm (OD600) at 6‐h intervals. Experiments were performed with three biological replicates.
Fig. S2 Exopolysaccharide production of Xanthomonas oryzae pv. oryzae (Xoo) strains. Extracellular polysaccharide (EPS) secretion in Xoo strains was detected by examination of the colony and quantification of EPS production. Values represent the average of three independent experiments, and the error bars indicate the standard deviations.
Fig. S3 Biofilm formation of Xanthomonas oryzae pv. oryzae (Xoo) strains. Biofilm formation in Xoo strains was quantified. The values represent the average of three independent experiments, and the error bars indicate the standard deviations.
Fig. S4 Sequence alignment analysis of glycosyltransferase from Xanthomonas oryzae pv. oryzae PXO99A and Xanthomonas campestris pv. campestris ATCC33913. The sequences were downloaded from the National Center for Biotechnology Information (NCBI) website, and sequence alignment was performed using DNAMAN software. The shading indicates identical amino acids.
Table S1 Primers used in this study


