Summary
Cyclodipeptides, formed from two amino acids by cyclodehydration, are produced naturally by many organisms, and are known to possess a large number of biological activities. In this study, we found that cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro) (where Pro is proline) could induce defence responses and systemic resistance in Nicotiana benthamiana. Treatment with the two cyclodipeptides led to a reduction in disease severity by Phytophthora nicotianae and Tobacco mosaic virus (TMV) infections compared with controls. Both cyclopeptides triggered stomatal closure, induced reactive oxygen species production and stimulated cytosolic calcium ion and nitric oxide production in guard cells. In addition, the application of cyclodipeptides significantly up‐regulated the expression of the plant defence gene PR‐1a and the PR‐1a protein, and increased cellular salicylic acid (SA) levels. These results suggest that the SA‐dependent defence pathway is involved in cyclodipeptide‐mediated pathogen resistance in N. benthamiana. We report the systemic resistance induced by cyclodipeptides, which sheds light on the potential of cyclodipeptides for the control of plant diseases.
Keywords: cyclodipeptides, cyclo (d‐Pro‐d‐Pro), cyclo (l‐Pro‐l‐Pro), disease resistance, Nicotiana benthamiana, salicylic acid, SAR
Introduction
Plants have evolved a complex immune system to protect themselves against the invasion of a broad array of environmental microorganisms (Zhang et al., 2010; Zuppini et al., 2004). In the early phase of plant immunity, the defence response is induced by pathogen‐associated molecular patterns (PAMPs) and pathogen elicitors (Chen et al., 2015).
Elicitors secreted by phytopathogens include a variety of compounds belonging to different chemical families: proteins, glycoproteins, glycans, lipids and synthetic molecules. Elicitors may be constituents of the pathogen or may be secreted by the pathogen, or may be substances released by hydrolytic enzymes produced by pathogens and plants (Zhang et al., 2009). The recognition of an elicitor by the plant cell is followed by calcium ion influx and the production of active oxygen species (AOS) and nitric oxide (NO), which regulate many processes, interconnecting branch pathways that amplify and specify the physiological response (such as the hypersensitive response and stomatal closure) through transcriptional and metabolic changes (Garcia‐Brugger et al., 2006; Zhang et al., 2010). In particular, the early activation of genes involved in phytohormone biosynthesis modifies the hormonal balance, thus activating distinct defence pathways that depend on different regulators (Ton et al., 2002).
Cyclodipeptides (diketopiperazines, DKPs), produced by the condensation of two α‐amino acids, are one of the smallest and simplest type of peptide compound, and have been found in many known microorganisms in nature, such as species in the genera Pseudomonas, Bacillus, Burkholderia and Aspergillus (Borthwick, 2012; Holden et al., 1999; Nishanth Kumar et al., 2013). Owing to the stable framework of the six‐member ring structure, two hydrogen‐bond donors and two hydrogen‐bond receptors, cyclodipeptides exhibit a broad range of biological activities, such as antitumor (Nicholson et al., 2006), antifungal (Nishanth Kumar et al., 2013), antibacterial (Rhee, 2004) and antiviral (Kwak et al., 2013) activity. In addition, Holden et al. (1999) have shown that several Gram‐negative bacteria produce and secrete cyclodipeptides with the ability to activate or antagonize other LuxR‐based quorum‐sensing systems. Ortiz‐Castro et al. (2011) have reported that three cyclodipeptides from Pseudomonas aeruginosa are involved in plant growth promotion, which is indicative of an auxin‐like activity.
N‐Acyl‐l‐homoserine lactone (AHL) signal molecules have been demonstrated to play a role in the biocontrol activity of rhizobacteria in plants through the induction of systemic resistance to pathogens (Schuhegger et al., 2006). In addition, Chen et al. (2015) have shown recently that cyclic dipeptides produced by the fungus Eupenicillium brefeldianum HMP‐F96 induce extracellular alkalinization and H2O2 production in tobacco cell suspensions, although this study did not further investigate the defence genes and pathways activated by the cyclodipeptides.
The aim of the present study was to investigate the effect of cyclodipeptides on the plant defence response. For this purpose, eight synthetic cyclodipeptides were compared in terms of their effects on induced systemic resistance in Nicotiana benthamiana Domin to the phytopathogens Phytophthora nicotianae Breda de Haan (the cause of black shank disease of tobacco) and the tobamovirus Tobacco mosaic virus (TMV). We then examined the cyclodipeptides for their abilities to interfere with the physiological responses and plant signalling pathways. In addition, we analysed the expression of plant defence genes and phytohormone levels following treatment with cyclodipeptides. Our results provide conclusive evidence that cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro) (where Pro is proline) can induce resistance against pathogen infection in N. benthamiana through the activation of the salicylic acid (SA)‐mediated plant defence pathway.
Results
Cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro) treatments induce resistance in N. benthamiana plants against P. nicotianae and TMV infections
To investigate whether cyclodipeptides have the ability to induce systemic resistance in plants, leaves of N. benthamiana were infiltrated with cyclodipeptides at a single spot on one half of the leaf, and the opposite side was then inoculated with P. nicotianae. The disease symptoms were assessed 48 h after P. nicotianae inoculation by comparing the sizes of the lesions. Typical water‐soaked Phytophthora lesions were observed on the control leaves at 48 h post‐inoculation. Pretreatment with cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro) significantly inhibited the expansion of lesions in inoculated leaves of N. benthamiana (inhibition rate 57.32%–59.25%; P < 0.01), although other cyclodipeptides had no significant effect (Fig. 1). Resistance was also evaluated by inoculation with TMV. The number of lesions and amount of virus were measured 7 days after inoculation. We observed a similar phenomenon to the P. nicotianae inoculation, in that there was a remarkable reduction (P < 0.01) in the number of lesions and amount of virus in N. benthamiana leaves after cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro) treatments, whereas the other treatments showed no significant difference compared with the controls (Table 1). Together, these results indicate that cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro) are effective at inducing systemic pathogen resistance in N. benthamiana.
Figure 1.
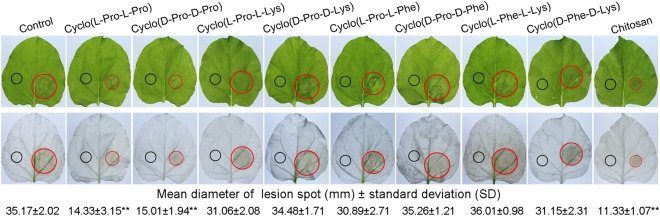
Cyclo (l‐Pro‐l‐Pro)‐ and cyclo (d‐Pro‐d‐Pro)‐induced resistance in Nicotiana benthamiana to Phytophthora nicotianae. Fully expanded leaves collected 4 h after cyclodipeptide treatments (black circle) were inoculated with a 7 mm × 7 mm P. nicotianae hyphal plug on the right side of the upper leaf surfaces opposite the cyclodipeptide‐infiltrated left sides. The leaves were then placed in Petri dishes containing filter paper saturated with sterilized distilled water and kept under a 16 h day/8 h night regime at 25°C. Photographs of the lesions were taken at 48 h post‐inoculation, and the lesion diameters (red circles) were measured. Both the original and ethanol‐bleached images are shown. Resistance evaluation was based on the diameter of the lesions. Data are means ± standard deviations (SDs) from five experiments. **Highly significant difference compared with the control (P < 0.01). Lys, lysine; Phe, phenylalanine; Pro, proline.
Table 1.
Response of Nicotiana benthamiana treated with cyclodipeptides and chitosan to inoculation with Tobacco mosaic virus (TMV).
| Treatment | TMV disease severity | TMV detection by ELISA (450 nm) |
|---|---|---|
| Control | 177 ± 6.5 | 1.603 ± 0.0512 |
| Cyclo (l‐Pro‐l‐Pro) | 67 ± 8.76* | 1.021 ± 0.0241* |
| Cyclo (d‐Pro‐d‐Pro) | 63 ± 11.59* | 0.965 ± 0.0438* |
| Cyclo (l‐Pro‐l‐Lys) | 164 ± 5.03 | 1.581 ± 0.0732 |
| Cyclo (d‐Pro‐d‐Lys) | 162 ± 10.41 | 1.521 ± 0.0598 |
| Cyclo (l‐Pro‐l‐Phe) | 158 ± 20.29 | 1.483 ± 0.0916 |
| Cyclo (d‐Pro‐d‐Phe) | 170 ± 13.24 | 1.610 ± 0.0261 |
| Cyclo (l‐Phe‐l‐Lys) | 175 ± 4.51 | 1.567 ± 0.0615 |
| Cyclo (d‐Phe‐d‐Lys) | 156 ± 11.54 | 1.492 ± 0.0374 |
| Chitosan | 58 ± 6.91* | 0.873 ± 0.0487* |
Lys, lysine; Phe, phenylalanine; Pro, proline.
TMV disease severity was determined by the number of visible viral lesions 7 days after inoculation. The amount of virus in inoculated leaves was also assayed by enzyme‐linked immunosorbent assay (ELISA). Data are expressed as means ± standard deviation (SD).
*Indicates a highly significant difference compared with the control (P < 0.01).
Effects of cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro) on stomatal closure in N. benthamiana leaves
Stomata are specialized epidermal structures, consisting of a pore surrounded by two guard cells, which regulate the loss of water to the atmosphere and the entry of carbon dioxide (CO2) into the plants for photosynthesis (Chen et al., 2004). It has been reported previously that elicitors, such as chitosan, can induce stomatal closure (Lee et al., 1999), and guard cells exhibit a classic innate immune response to both PAMP compounds and pathogens (Zhang et al., 2009). Stomatal responses to cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro) were observed in N. benthamiana leaves. Following 3 h of treatment, both cyclodipeptides induced stomatal closure, and the aperture size was clearly reduced relative to the control (P < 0.01), as illustrated in Fig. 2.
Figure 2.
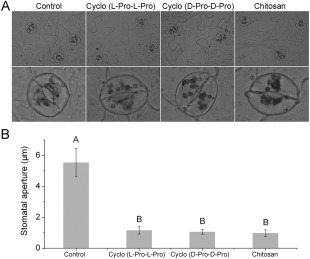
Cyclo (l‐Pro‐l‐Pro)‐ and cyclo (d‐Pro‐d‐Pro)‐induced stomatal closure in leaves of Nicotiana benthamiana. Photographs of leaf epidermal peels were taken after 3 h of incubation in control buffer, cyclo (l‐Pro‐l‐Pro) (50 μm), cyclo (d‐Pro‐d‐Pro) (50 μm) and chitosan (100 μg/mL). Experiments were repeated three times, and representative images are shown in (A) (bottom panels, enlarged images). Stomatal aperture measurements are shown in (B). Values represent means ± standard deviations (SDs) (n = 50) from three independent experiments; different letters indicate a highly significant difference (P < 0.01). Pro, proline.
H2O2 accumulation by guard cells in response to cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro)
Numerous studies have shown that H2O2 is involved in elicitor‐inhibited stomatal opening and elicitor‐enhanced stomatal closure (Lee et al., 1999). To investigate whether H2O2 also participates in cyclo (l‐Pro‐l‐Pro)‐ and cyclo (d‐Pro‐d‐Pro)‐induced stomatal closure, we measured H2O2 production using diaminobenzidine (DAB) staining. Figure 3A shows the development of the DAB–H2O2 reaction product in N. benthamiana leaves 6 h after treatment. A heavy brown precipitate was observed in plants after treatment with the two cyclodipeptides compared with the control (Fig. 3A), which was consistent with the results of further quantitative analysis using the software Quantity One (Fig. 3B).
Figure 3.
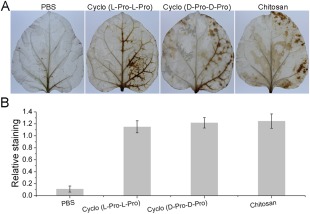
Detection of hydrogen peroxide in Nicotiana benthamiana leaves using diaminobenzidine (DAB) staining. (A) Photographs of representative ethanol‐bleached leaves 6 h after phosphate‐buffered saline (PBS) (10 mm), cyclo (l‐Pro‐l‐Pro) (50 μm), cyclo (d‐Pro‐d‐Pro) (50 μm) and chitosan (100 μg/mL) treatments. (B) Quantitative scoring of staining in treated leaves. Different letters indicate that the means are statistically significantly different at P < 0.01. Pro, proline.
Cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro) induce increases in Ca2+, NO and AOS in guard cells
H2O2 production has been reported to reduce the size of the stomatal aperture by activating plasma membrane‐localized calcium channels, leading to cytosolic Ca2+ elevation (Chen et al., 2004; Zhang et al., 2009). Therefore, to evaluate the relative contributions of NADPH oxidases, and whether cyclodipeptide‐induced H2O2 production has an effect on [Ca2+]cyt increase, calcium fluorescence imaging analysis of guard cells from intact epidermal strips was conducted using the dye 1‐[2‐amino‐5‐(2,7‐dichloro‐6‐hydroxy‐3‐oxo‐9‐xanthenyl)phenoxy]‐2‐(2‐amino‐5‐methylphenoxy)ethane‐N,N′,N′‐tetraacetic acid, pentaacetoxymethyl ester (fluo‐3AM). We observed almost no fluorescence in control‐treated guard cells, whereas application of cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro) resulted in guard cells that showed obvious Ca2+ fluorescence (Fig. 4A,B). Further quantification showed that no significant differences were observed between cyclodipeptides and the elicitor chitosan (Fig. 4C).
Figure 4.
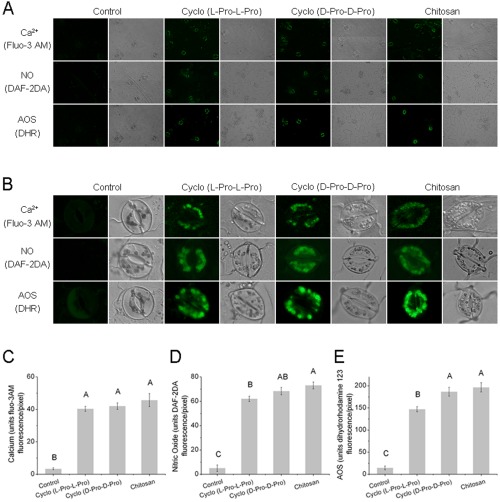
Cyclo (l‐Pro‐l‐Pro)‐ and cyclo (d‐Pro‐d‐Pro)‐induced intracellular Ca2+, nitric oxide (NO) and active oxygen species (AOS) accumulation in guard cells of Nicotiana benthamiana. The calcium fluorescence probe 1‐[2‐amino‐5‐(2,7‐dichloro‐6‐hydroxy‐3‐oxo‐9‐xanthenyl)phenoxy]‐2‐(2‐amino‐5‐methylphenoxy)ethane‐N,N ′,N ′‐tetraacetic acid, pentaacetoxymethyl ester (fluo‐3AM), the NO‐sensitive dye 4,5‐diaminofluorescein diacetate (DAF‐2DA) and the AOS dye dihydrorhodamine 123 (DHR) were loaded into cells of the epidermal peels. Fluorescence was measured after incubation with control buffer, cyclo (l‐Pro‐l‐Pro) (50 μM), cyclo (d‐Pro‐d‐Pro) (50 μM) or chitosan (100 μg/mL). For each treatment, fluorescence and bright‐field images are shown. Experiments were repeated at least three times, and representative images are shown in (A) and (B) (enlarged images). Quantitative analyses of in vivo Ca2+, NO and AOS generation are shown in (C–E). Results are presented as means ± standard deviation (SD) (n > 3). Different letters indicate means that show highly statistically significant differences at P < 0.01. Pro, proline.
NO coordinates plant innate immunity by serving as a cellular signalling molecule in a wide range of organisms including plants, especially in stomatal guard cells (Ali et al., 2007; Asai and Yoshioka, 2009; Zhang et al., 2010). Rapid production of AOS, including H2O2 and other species (superoxide, the highly reactive hydroxyl radical), is characteristic of the plant resistance response, and AOS are thought to act as second messengers in the induction of resistance reactions, such as the expression of defence genes (Lee et al., 1999; Pasqualini et al., 2007). Our study employed the specific fluorescent dyes 4,5‐diaminofluorescein diacetate (DAF‐2DA) and dihydrorhodamine 123 (DHR) to monitor NO and AOS generation in vivo. All epidermal peels showed bright fluorescence after treatment with cyclodipeptides. In contrast, the control guard cells showed almost no fluorescence (Fig. 4A,B), indicating that cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro) have a stimulating effect on NO and AOS accumulation. This conclusion was supported by the enhanced transcription of two genes responsible for nitrate reductase (NIA1 and NIA2) and two genes responsible for NADPH oxidase (rbohA and rbohB), as determined by quantitative real‐time reverse transcription‐polymerase chain reaction (qRT‐PCR) (Fig. S1, see Supporting Information).
Cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro) activate plant defence genes through the SA‐mediated signalling pathway
Plants utilize a broad range of defence mechanisms to prevent invasion by pathogens, and systemic acquired resistance (SAR), associated with the accumulation of SA, and induced systemic resistance (ISR), dependent on the jasmonic acid (JA) and ethylene (ET) pathways, are two different defence mechanisms that are induced by pathogens or non‐pathogens (Schuhegger et al., 2006; Zhu et al., 2014). To investigate which signalling pathway is involved in the resistance of N. benthamiana to pathogens induced by cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro), we assayed the expression of the SA‐responsive gene PR‐1a, the JA synthesis‐related gene LOX and the ET‐responsive gene ERF1. Reverse transcription‐polymerase chain reaction (RT‐PCR) and qRT‐PCR showed increased expression of PR‐1a (10‐fold change) after 6 h of treatment, whereas expression of LOX and ERF1 was not significantly up‐regulated (Figs 5A,B and S2, see Supporting Information). Results of western blot analysis for the PR‐1a protein were consistent, and showed increased accumulation of the protein (Fig. 5C). Furthermore, we measured the levels of the signal molecule SA in leaves of N. benthamiana. Both free and conjugated SA levels were elevated between five‐ and eight‐fold after treatment with the cyclodipeptides (Fig. 5D). The JA and ET contents were quantified by high‐performance liquid chromatography‐mass spectrometry (HPLC‐MS), and showed no significant increases (Fig. S3, see Supporting Information). Furthermore, transgenic NahG tobacco plants, which are unable to accumulate SA and develop SAR, were treated with the cyclodipeptides prior to inoculation with the pathogens. Cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro) did not induce disease resistance according to the number and size of the lesions and PR‐1a expression in NahG plants (Fig. S4, see Supporting Information). Our results therefore suggest that the SA signalling pathway is induced during treatment of N. benthamiana with cyclodipeptides.
Figure 5.
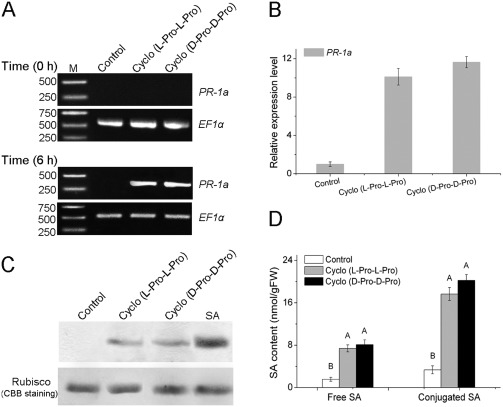
Induction of markers for the plant defence response following inoculation with cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro). (A) Reverse transcription‐polymerase chain reaction (RT‐PCR) analysis to examine mRNA levels of the defence‐related gene PR‐1a in Nicotiana benthamiana leaves. Equal loads of cDNA were quantified by amplification of the constitutively expressed gene EF1α. (B) Quantitative real‐time PCR analysis of PR‐1a gene expression in response to cyclodipeptide treatments. Values were normalized to the levels of EF‐1α. The y axis represents the mean expression level (fold‐change) ± standard deviation (SD) (n = 3) relative to the control. (C) Western blot analysis of PR‐1a protein levels in N. benthamiana leaves. Salicylic acid (SA) was used as the positive control treatment. Rubisco stained with Coomassie Brilliant Blue (CBB) R‐250 is shown as an internal control. (D) Contents of free and conjugated SA in N. benthamiana leaves after treatment with cyclodipeptides. The different letters indicate highly significant differences at P < 0.01. FW, fresh weight; Pro, proline.
Discussion
The roles of cyclodipeptides in biological processes have been reported extensively in animal systems (Borthwick, 2012), but whether these molecules can function as elicitors in the plant defence response to pathogens is unclear. In this study, two cyclodipeptides, cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro), were shown to be able to induce systemic pathogen resistance in N. benthamiana. Although the number of synthetic compounds is insufficient to obtain robust structure–function relationships, the tricyclic ring skeleton could be crucial for the resistance‐inducing activity of the cyclodipeptides. This is consistent with previous findings indicating that the tricyclic hydroxyproline‐containing DKPs are better plant growth regulators and resistance inducers to abiotic stress (drought) in plants (Chen et al., 2015).
NO and H2O2 have emerged as ubiquitous components of signal transduction pathways that control a diverse range of physiological functions in a wide spectrum of biological systems. Cytosolic Ca2+ levels quickly increase on pathogen infection, and Ca2+ influx is necessary for AOS production after elicitation. Stomatal closure occurs in response to physiological and stress stimuli (Bright et al., 2006, Garcia‐Brugger et al., 2006). Numerous studies have demonstrated that elicitors can induce the oxidative burst, which can then limit the spread of invading pathogens by generating AOS, inducing NO‐associated stomatal closure and triggering a Ca2+ spike (Zhang et al., 2009, 2010). Cyclodipeptides have also been shown to induce steady increases in H2O2 production in tobacco cells during exposure, which level off thereafter (Chen et al., 2015). In the present study, we found that guard cells can recognize cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro), can generate H2O2 and can then respond to H2O2 by the narrowing of stomatal apertures. Furthermore, we confirmed that these two cyclodipeptides can stimulate the accumulation of AOS and NO, and also cause an increase in the cytosolic Ca2+ concentration. These observations suggest the possibility that cyclodipeptides can be considered to be potential candidates for a new group of general elicitors for plant defence.
Disease resistance in plants is a highly regulated phenomenon that depends on several signalling pathways, each of which is activated by a different set of biotic and abiotic stimuli (Schuhegger et al., 2006). Pretreatment with elicitors generally renders a plant more resistant to subsequent pathogen attack, and elicitor stimulation has been demonstrated to induce plant defence responses, such as the hypersensitive response, pathogenesis‐related (PR) gene expression and cell wall stabilization (Lee et al., 1999). Arachis hypogaea (peanut) leaves treated with chitosan, a well‐studied elicitor, show an increase in endogenous SA levels, as well as an increase in the activities of intercellular chitinase and glucanase (Sathiyabama and Balasubramanian, 1998). AHL has been shown to systemically induce SA‐ and ethylene‐dependent defence genes (PR‐1a, 26‐kDa acidic and 30‐kDa basic chitinases) by macroarray and northern blot analyses (Schuhegger et al., 2006). In our study, SA, one of the most well‐characterized plant signalling molecules, appears to be involved in systemic resistance against pathogens induced by cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro), because the expression of PR‐1a, which is considered to be a marker for the SA‐dependent defence pathway SAR (Asai and Yoshioka, 2009), is rapidly up‐regulated following cyclodipeptide treatment. In addition, SA accumulates and levels of the PR‐1a protein increase, as shown by western blot analysis. A similar dependence on the SA signalling pathway has also been evaluated in Arabidopsis and rice (Fig. S5, see Supporting Information).
In conclusion, the present study provides direct evidence for the systemic induction of pathogen resistance in N. benthamiana by cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro). Both cyclodipeptides induce SA systemically and lead to enhanced gene expression of typical defence‐related proteins, which are associated with the establishment of SAR in plants Therefore, cyclodipeptides may find important applications in plant disease management strategies in the future.
Experimental Procedures
Plant material and growth conditions
Seeds of N. benthamiana were surface sterilized in 95% (v/v) ethanol for 5 min, followed by a 5% (w/v) solution of sodium hypochlorite for 5 min. After five washes with sterile distilled water, seeds were allowed to germinate on solid Murashige and Skoog medium (Murashige and Skoog, 1962). Seedlings were then transferred to pots containing sterilized vermiculite at a density of one per pot. Seedlings were incubated in a controlled environment growth chamber under a 16 h/8 h light/dark cycle at 25 ºC.
Chemical compounds
The cyclodipeptides used in this study (Fig. S6, see Supporting Information) were synthesized by the Chinese Peptide Company (Hangzhou, China). For each compound, the structure of the synthetic material was confirmed by MS and proton nuclear magnetic resonance (NMR) spectroscopy. Chitosan was purchased from Sigma‐Aldrich (St. Louis, MO, USA) and solubilized in 0.5 m aqueous acetic acid, after which the solution was adjusted to pH 5.2 with 1 M KOH (Zuppini et al., 2004). Chitosan was used as a positive control in the experiments.
Evaluation of the effect of the cyclodipeptides on the disease severity of P. nicotianae and TMV
Based on the method described by Teng et al. (2014), one half (left side) of a leaf from N. benthamiana was infiltrated with either phosphate‐buffered saline (PBS, 10 mm) or a cyclodipeptide solution (50 μm). Detached leaves were then transferred to Petri dishes containing sterile water‐saturated filter paper after 4 h. A 7 mm × 7 mm hyphal plug of P. nicotianae was placed on the surface of the right side of each leaf, and the samples were kept in the dark at 25°C. Disease symptoms were recorded after 48 h of incubation, the leaves were fixed in 100% ethanol and the resistance rate was calculated based on the measurement of the diameter of the P. nicotianae lesion using the following formula: inhibition rate = (diameter of control – diameter of treatment)/diameter of control × 100%.
TMV was maintained by mechanical passage in a temperature‐controlled glasshouse, as described by Luo et al. (2010). Prior to inoculation, cyclodipeptides or sterile water were sprayed on the lower leaves of the plants. After 4 days, the plants were inoculated with TMV by rubbing the untreated upper leaves with carborundum (500 mesh). The number of visible viral lesions was then counted 7 days after inoculation. Simultaneously, enzyme‐linked immunosorbent assays (ELISAs) were performed to quantify the amount of virus in the inoculated leaf samples, according to Wang et al. (2011).
Stomatal aperture measurements
Stomatal apertures were measured as described by Chen et al. (2004). Nicotiana benthamiana leaf strips were floated with the abaxial (lower) epidermis contacting MES buffer (10 mm MES‐Tris, 5 mm KCl and 50 mm CaCl2, pH 6.15) in the light for 2–3 h to open the stomata fully in order to minimize the effects of other factors in the stomatal response. The epidermal strips were then treated with either 50 μm cyclo (L‐Pro‐L‐Pro) or cyclo (D‐Pro‐D‐Pro) for 3 h to induce a stomatal response. Stomatal aperture images on the strips were captured with an Olympus BX43 microscope using cellSens Standard Software (Tokyo, Japan). Stomatal aperture diameters were measured from 50 randomly selected stomata. Each assay was repeated three times.
DAB staining
Following the method of Samuel et al. (2005), leaves collected 6 h after treatment were incubated in PBS buffer (pH 7.4) containing 0.5% (w/v) DAB for 8 h at 25 ºC in the light. The leaves were then boiled in 96% ethanol for 10 min to remove the dye. Brown precipitates were observed in the leaves, indicating the presence of an H2O2 burst. The intensity and pattern of H2O2 staining in the leaves were analysed using Quantity One software (Bio‐Rad, Milan, Italy).
Measurement of Ca2+, NO and AOS in guard cells
Ca2+ levels were measured in guard cells as described by Chen et al. (2004). The N. benthamiana leaf epidermal strips were incubated in 10 mm fluo‐3AM in loading buffer (10 mm MES‐Tris, pH 6.15) at 4 ºC for 2 h in darkness. The strips were then kept at room temperature for 1 h after rinsing three times with MES buffer. Guard cell images were taken 3 h after treatment using a model LSM710 confocal laser scanning microscope (CLSM; Carl Zeiss, Jena, Germany).
The fluorophore DAF‐2DA (Sigma‐Aldrich) was used to analyse NO accumulation in guard cells according to Ali et al. (2007). Following Zhang et al. (2009), AOS production was determined using DHR (Merck, Whitehouse Station, NJ, USA)
RNA isolation, RT‐PCR and qRT‐PCR
Total RNA was extracted using a Plant RNA Kit (Omega Bio‐Tek, Norcross, GA, USA) according to the manufacturer's instructions. First‐strand cDNA was synthesized using Reverse Transcriptase (TaKaRa Bio Inc., Dalian, China) with oligo dT primers, and the resulting cDNA was used as the template for subsequent PCR amplification. RT‐PCR products were resolved on an agarose gel to determine the expression levels of the target genes. qRT‐PCR was performed using SYBR Premix Ex Taq (TaKaRa Bio Inc.) on a 7500 Fast Real‐Time PCR Detection System (Applied Biosystems, Foster, CA USA). Expression of the N. benthamiana EF1α gene was used to normalize the gene expression in each sample. Gene‐specific PCR primers are shown in Table S1 (see Supporting Information).
Western blotting and SA determination
Western blot analysis was performed as described previously (Takakura et al., 2004). Briefly, proteins were separated by sodium dodecylsulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE), blotted onto a polyvinylidene difluoride (PVDF) membrane (Millipore, Bed‐ford, MA, USA), probed with a polyclonal antibody raised against the tobacco PR‐1a protein as primary antibody, and finally visualized using DAB after incubation with a secondary antibody [goat anti‐rabbit immunoglobulin G‐horseradish peroxidase (IgG‐HRP)].
Phytohormones and free and conjugated SA were extracted from leaves and roots and quantified according to the method of Schuhegger et al. (2006).
Statistical analyses
Each experiment was conducted with a minimum of three independent replications. The data were analysed by analysis of variance (ANOVA), followed by Fisher's least‐significant difference test (P < 0.01), using SPSS software (SPSS Inc., Chicago, IL, USA).
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Table S1 Oligo DNA primers used in this study.
Fig. S1 Relative expression levels of genes associated with nitric oxide (NO) and active oxygen species (AOS) accumulation. Nicotiana benthamiana leaves were harvested after 3 h of treatment with the cyclodipeptides cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro). mRNA levels of the NIA1, NIA2 (A) and robhA, robhB (B) genes were quantified by quantitative real‐time reverse transcription‐polymerase chain reaction (qRT‐PCR). Values are expressed as means ± standard deviation (SD). Different letters indicate means that show highly statistically significant differences at P < 0.01.
Fig. S2 Expression analysis of the defence‐related genes LOX and ERF1 in Nicotiana benthamiana after treatment with the cyclodipeptides cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro). Values are expressed as means ± standard deviation (SD).
Fig. S3 Jasmonic acid (JA) and ethylene (ET) levels in Nicotiana benthamiana leaves in response to treatment with the cyclodipeptides cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro). JA was quantified by gas chromatography‐mass spectrometry (GC‐MS), and 100 ng of 9,10‐dihydrojasmonic acid was added per gram of fresh weight as an internal standard. Ethylene (ET) production was measured by GC after the leaves had been weighed and placed in 25‐mL gas‐tight serum flasks.
Fig. S4 Effects of the cyclodipeptides cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro) on pathogenesis‐related (PR) gene expression and disease resistance in transgenic NahG tobacco plants. (A) The disease severity of cyclodipeptide‐treated transgenic NahG tobacco plants following pathogen infection. (B) Transcription patterns of PR‐1a in NahG tobacco plants after cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro) treatments.
Fig. S5 Transcription patterns of AtPR‐1a, AtLOX and AtERF1 genes in Arabidopsis, and OsPR‐1a, OsLOX and OsERF1 genes in rice, after cyclodipeptide treatments.
Fig. S6 Structures of the eight cyclodipeptides synthesized and evaluated in this study.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (31471811), the Agro‐Scientific Research in the Public Interest (20130315), the Key R&D Program of Jiangsu Province (BE2015354) and the National High‐Tech R&D Program of China (2012AA101504).
References
- Ali, R. , Ma, W. , Lemtiri‐Chlieh, F. , Tsaltas, D. , Leng, Q. , von Bodman, S. and Berkowitz, G.A. (2007) Death don't have no mercy and neither does calcium: Arabidopsis CYCLIC NUCLEOTIDE GATED CHANNEL2 and innate immunity. Plant Cell, 19, 1081–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai, S. and Yoshioka, H. (2009) Nitric oxide as a partner of reactive oxygen species participates in disease resistance to necrotrophic pathogen Botryis cinerea in Nicotiana benthamiana . Mol. Plant–Microbe Interact. 22, 619–629. [DOI] [PubMed] [Google Scholar]
- Borthwick, A.D. (2012) 2,5‐Diketopiperazines: synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem. Rev. 112, 3641–3716. [DOI] [PubMed] [Google Scholar]
- Bright, J. , Desikan, R. , Hancock, J.T. , Weir, I.S. and Neill, S.J. (2006) ABA‐induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 45, 113–122. [DOI] [PubMed] [Google Scholar]
- Chen, Y.L. , Huang, R.F. , Xiao, Y.M. , Lu, P. , Chen, J. and Wang, X.C. (2004) Extracellular calmodulin‐induced stomatal closure is mediated by heterotrimeric G protein and H2O2 . Plant Physiol. 136, 4096–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. , Mou, Y. , Ling, J. , Wang, N. , Wang, X. and Hu, J. (2015) Cyclic dipeptides produced by fungus Eupenicillium brefeldianum HMP‐F96 induced extracellular alkalinization and H2O2 production in tobacco cell suspensions. World J. Microbiol. Biotechnol. 31, 247–253. [DOI] [PubMed] [Google Scholar]
- Garcia‐Brugger, A. , Lamotte, O. , Vandelle, E. , Bourque, S. , Lecourieux, D. , Poinssot, B. , Wendehenne, D. and Pugin, A. (2006) Early signaling events induced by elicitors of plant defenses. Mol. Plant–Microbe Interact. 19, 711–724. [DOI] [PubMed] [Google Scholar]
- Holden, M.T. , Ram Chhabra, S. , de Nys, R. , Stead, P. , Bainton, N.J. , Hill, P.J. , Manefield, M. , Kumar, N. , Labatte, M. , England, D. , Rice, S. , Givskov, M. , Salmond, G.P. , Stewart, G.S. , Bycroft, B.W. , Kjelleberg, S. and Williams, P. (1999) Quorum‐sensing cross talk: isolation and chemical characterization of cyclic dipeptides from Pseudomonas aeruginosa and other gram‐negative bacteria. Mol. Microbiol. 33, 1254–1266. [DOI] [PubMed] [Google Scholar]
- Kwak, M.K. , Liu, R. , Kwon, J.O. , Kim, M.K. , Kim, A.H. and Kang, S.O. (2013) Cyclic dipeptides from lactic acid bacteria inhibit proliferation of the influenza A virus. J. Microbiol. 51, 836–843. [DOI] [PubMed] [Google Scholar]
- Lee, S. , Choi, H. , Suh, S. , Doo, I.S. , Oh, K.Y. , Choi, E.J. , Schroeder Taylor, A.T. , Low, P.S. and Lee, Y. (1999) Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen species from guard cells of tomato and Commelina communis . Plant Physiol. 121, 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, Y. , Zhang, D.D. , Dong, X.W. , Zhao, P.B. , Chen, L.L. , Song, X.Y. , Wang, X.J. , Chen, X.L. , Shi, M. and Zhang, Y.Z. (2010) Antimicrobial peptaibols induce defense responses and systemic resistance in tobacco against tobacco mosaic virus. FEMS Microbiol. Lett. 313, 120–126. [DOI] [PubMed] [Google Scholar]
- Murashige, T. and Skoog, F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant, 15, 473–497. [Google Scholar]
- Nicholson, B. , Lloyd, G.K. , Miller, B.R. , Palladino, M.A. , Kiso, Y. , Hayashi, Y. and Neuteboom, S.T. (2006) NPI‐2358 is a tubulin‐depolymerizing agent: in‐vitro evidence for activity as a tumor vascular‐disrupting agent. Anticancer Drugs, 17, 25–31. [DOI] [PubMed] [Google Scholar]
- Nishanth Kumar, S. , Mohandas, C. and Nambisan, B. (2013) Purification of an antifungal compound, cyclo(L‐Pro‐D‐Leu) for cereals produced by Bacillus cereus subsp. thuringiensis associated with entomopathogenic nematode. Microbiol. Res. 168, 278–288. [DOI] [PubMed] [Google Scholar]
- Ortiz‐Castro, R. , Díaz‐Pérez, C. , Martínez‐Trujillo, M. , del Río, R.E. , Campos‐García, J. and López‐Bucio, J. (2011) Transkingdom signaling based on bacterial cyclodipeptides with auxin activity in plants. Proc. Natl. Acad. Sci. USA, 108, 7253–7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualini, S. , Paolocci, F. , Borgogni, A. , Morettini, R. , and Ederli, L. (2007) The overexpression of an alternative oxidase gene triggers ozone sensitivity in tobacco plants. Plant Cell Environ. 30, 1545–1556. [DOI] [PubMed] [Google Scholar]
- Rhee, K.H. (2004) Cyclic dipeptides exhibit synergistic, broad spectrum antimicrobial effects and have anti‐mutagenic properties. Int. J. Antimicrob. Agents, 24, 423–427. [DOI] [PubMed] [Google Scholar]
- Samuel, M.A. , Hall, H. , Krzymowska, M. , Drzewiecka, K. , Hennig, J. and Ellis, B.E. (2005) SIPK signaling controls multiple components of harpin‐induced cell death in tobacco. Plant J. 42, 406–416. [DOI] [PubMed] [Google Scholar]
- Sathiyabama, M. , and Balasubramanian, R. (1998) Chitosan induces resistance components in Arachis hypogaea against leaf rust caused by Puccinia arachidis Speg. Crop Prot. 17, 307–313. [Google Scholar]
- Schuhegger, R. , Ihring, A. , Gantner, S. , Bahnweg, G. , Knappe, C. , Vogg, G. , Hutzler, P. , Schmid, M. , Van Breusegem, F. , Eberl, L. , Hartmann, A. , and Langebartels, C. (2006) Induction of systemic resistance in tomato by N‐acyl‐L‐homoserine lactone‐producing rhizosphere bacteria. Plant Cell Environ. 29, 909–918. [DOI] [PubMed] [Google Scholar]
- Takakura, Y. , Ishida, Y. , Inoue, Y. , Tsutsumi, F. and Kuwata, S. (2004) Induction of a hypersensitive response‐like reaction by powdery mildew in transgenic tobacco expressing harpinpss . Physiol. Mol. Plant Pathol. 64, 83–89. [Google Scholar]
- Teng, W. , Zhang, H. , Wang, W. , Li, D. , Wang, M. , Liu, J. , Zhang, H. , Zheng, X. and Zhang, Z. (2014) ALY proteins participate in multifaceted Nep1Mo‐triggered responses in Nicotiana benthamiana and Arabidopsis thaliana . J. Exp. Bot. 65, 2483–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton, J. , Van Pelt, J.A. , Van Loon, L.C. and Pieterse, C.M. (2002) Differential effectiveness of salicylate‐dependent and jasmonate/ethylene‐dependent induced resistance in Arabidopsis . Mol. Plant–Microbe Interact. 15, 27–34. [DOI] [PubMed] [Google Scholar]
- Wang, S. , Wu, H.J. , Zhan, J. , Xia, Y.F. , Gao, S.F. , Wang, W.D. , Xue, P.Q. and Gao, X.W. (2011) The role of synergistic action and molecular mechanism in the effect of genetically engineered strain Bacillus subtilis OKBHF in enhancing tomato growth and cucumber mosaic virus resistance. BioControl, 56, 113–121. [Google Scholar]
- Zhang, H. , Fang, Q. , Zhang, Z. , Wang, Y. and Zheng, X. (2009) The role of respiratory burst oxidase homologues in elicitor‐induced stomatal closure and hypersensitive response in Nicotiana benthamiana . J. Exp. Bot. 60, 3109–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Dong, S. , Wang, M. , Wang, W. , Song, W. , Dou, X. , Zheng, X. and Zhang, Z. (2010) The role of vacuolar processing enzyme (VPE) from Nicotiana benthamiana in the elicitor‐triggered hypersensitive response and stomatal closure. J. Exp. Bot. 61, 3799–3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, F. , Xi, D.H. , Yuan, S. , Xu, F. , Zhang, D.W. and Lin, H.H. (2014) Salicylic acid and jasmonic acid are essential for systemic resistance against tobacco mosaic virus in Nicotiana benthamiana . Mol. Plant–Microbe Interact. 27, 567–577. [DOI] [PubMed] [Google Scholar]
- Zuppini, A. , Baldan, B. , Millioni, R. , Favaron, F. , Navazio, L. and Mariani, P. (2004) Chitosan induces Ca2+‐mediated programmed cell death in soybean cells. New Phytol. 161, 557–568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Table S1 Oligo DNA primers used in this study.
Fig. S1 Relative expression levels of genes associated with nitric oxide (NO) and active oxygen species (AOS) accumulation. Nicotiana benthamiana leaves were harvested after 3 h of treatment with the cyclodipeptides cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro). mRNA levels of the NIA1, NIA2 (A) and robhA, robhB (B) genes were quantified by quantitative real‐time reverse transcription‐polymerase chain reaction (qRT‐PCR). Values are expressed as means ± standard deviation (SD). Different letters indicate means that show highly statistically significant differences at P < 0.01.
Fig. S2 Expression analysis of the defence‐related genes LOX and ERF1 in Nicotiana benthamiana after treatment with the cyclodipeptides cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro). Values are expressed as means ± standard deviation (SD).
Fig. S3 Jasmonic acid (JA) and ethylene (ET) levels in Nicotiana benthamiana leaves in response to treatment with the cyclodipeptides cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro). JA was quantified by gas chromatography‐mass spectrometry (GC‐MS), and 100 ng of 9,10‐dihydrojasmonic acid was added per gram of fresh weight as an internal standard. Ethylene (ET) production was measured by GC after the leaves had been weighed and placed in 25‐mL gas‐tight serum flasks.
Fig. S4 Effects of the cyclodipeptides cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro) on pathogenesis‐related (PR) gene expression and disease resistance in transgenic NahG tobacco plants. (A) The disease severity of cyclodipeptide‐treated transgenic NahG tobacco plants following pathogen infection. (B) Transcription patterns of PR‐1a in NahG tobacco plants after cyclo (l‐Pro‐l‐Pro) and cyclo (d‐Pro‐d‐Pro) treatments.
Fig. S5 Transcription patterns of AtPR‐1a, AtLOX and AtERF1 genes in Arabidopsis, and OsPR‐1a, OsLOX and OsERF1 genes in rice, after cyclodipeptide treatments.
Fig. S6 Structures of the eight cyclodipeptides synthesized and evaluated in this study.


