Figure 3.
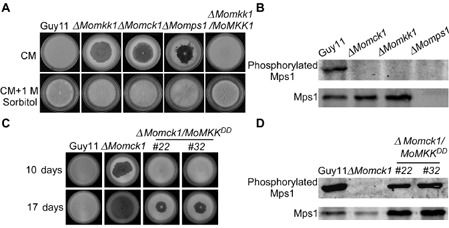
MoMkk1 functions upstream of MoMps1 and downstream of MoMck1. (A) Growth of wild‐type and mutant strains on complete medium (CM) without 1 m sorbitol (top). Growth of strains on CM with 1 m sorbitol (bottom). Consistent with ΔM omck1 and ΔM omps1, the ΔM omkk1 mutant also undergoes progressive autolysis on CM in the absence of osmotic stabilization. (B) Phosphorylation of MoMps1 in ΔM omkk1 and the other two cell wall integrity (CWI) pathway mutants. Immunoblots were performed with total proteins from hyphae of Guy11, ΔM omkk1, ΔM omck1 and ΔM omps1 mutants. The signal corresponding to phosphorylated MoMps1 was detected by binding of the antiphospho‐p44/42 antibody, with the Mpk1 antibody used as a control. MoMps1 phosphorylation in the ΔM omkk1 mutant was completely lost. (C) Autolysis observation. Wild‐type strain Guy11, the Δ M omck1 mutant and two transformants whose endogenous MKK 1 was replaced with MKK 1 T369D,T375D to obtain a constitutive activation of MoMps1 were allowed to grow on CM for 10 days. The strain with constitutively activated MoMps1 partially suppressed the autolysis of the Δ M omck1 mutant prior to 17 days of growth. (D) Phosphorylation of MoMps1 in two strains with constitutive activation of MoMps1 in the ΔM omck1 background. Proteins were prepared from mycelia cultured in liquid CM and the phosphorylated MoMps1 was detected by binding of the antiphospho‐p44/42 antibody, with the Mpk1 antibody as a control. The phosphorylation level of MoMps1 in the two transformants with constitutive activation of MoMps1 in the ΔM omck1 background indicated the activation of MoMps1.
