Figure 6.
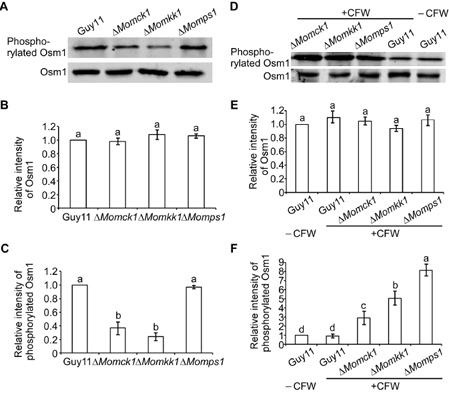
MoMkk1 is involved in the regulation of MoOsm1 phosphorylation in M agnaporthe oryzae. (A) Phosphorylation of MoOsm1 in Δ M omkk1, ΔM omck1 and ΔM omps1 mutants. Immunoblots of total hyphal proteins were obtained from Guy11, ΔM omkk1, ΔM omck1 and ΔM omps1 mutants. The intensity of the signal corresponding to phosphorylated MoOsm1 was detected by binding of the phosphorylated p38 (Thr180/Tyr182) antibody, with anti‐Hog1 antibody binding as control. (B, C) The intensities of the Western blotting bands were quantified with the ODYSSEY infrared imaging system (application software Version 2.1). The intensity of the MoOsm1 band in each mutant is relative to the amount of MoOsm1 in the wild‐type strain Guy11. Bars denote standard errors from three independent experiments. Values on the bars followed by the same letter were not significantly different at P = 0.05. (D) Phosphorylation of Osm1 in Guy11 and the three cell wall integrity (CWI) mutants with CFW treatment. The intensity of the signal corresponding to phosphorylated Osm1 was detected by binding of a phosphorylated p38 (Thr180/Tyr182) antibody, with an anti‐Hog1 antibody used as a control. (E, F) The intensity of the MoOsm1 band in each mutant is relative to the amount of MoOsm1 in the wild‐type strain Guy11. Bars denote standard errors from three independent experiments. Values on the bars followed by the same letter are not significantly different at P = 0.05.
