Figure 3.
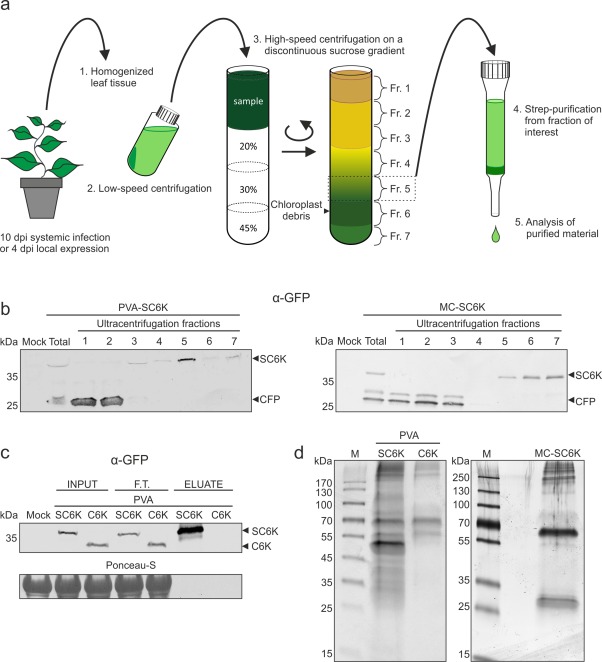
Purification of 6K2‐associated membranes from Potato virus A (PVA) infection. (a) A schematic representation of the purification protocol. PVA‐SC6K‐ and PVA‐C6K‐infected and SC6K‐expressing leaf tissues were homogenized and the cleared lysates were subjected to sucrose gradient centrifugation. Fraction 5 collected from the gradient was subjected to affinity purification via the 2×Strep‐tag. (b) The sucrose gradient fractions were analysed by Western blot analysis. SC6K concentrated to fraction 5 in the infection context and to fractions 5–7 when SC6K was expressed alone. (c) SC6K protein and its binding partners were subjected to 2×Strep‐tag affinity purification. SC6K protein was significantly enriched in the eluate. The 2× Strep‐tag‐specific purification was controlled with tag‐less C6K protein (F.T., flow through). (d) The outcome of the purification procedure was assessed by sodium dodecylsulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE), followed by silver staining, which revealed clear differences in the protein content between the purified PVA‐SC6K sample in the left panel and the controls: PVA‐C6K in the left panel and MC‐SC6K in the right panel.
