Figure 1.
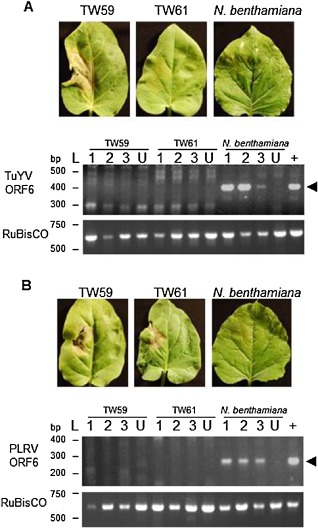
Nicotiana glutinosa is resistant to Turnip yellows virus (TuYV) and Potato leafroll virus (PLRV). Nicotiana glutinosa leaves of 4‐week‐old plants were agroinfected with Agrobacterium carrying the infectious clone of TuYV‐FL1. (A) Local infiltrated leaves of accession TW59 demonstrated a hypersensitive response (HR) against TuYV (left). Local leaves of TW61 (centre) and N. benthamiana (right, the susceptible control) appeared to be unresponsive. Representative leaves from three experiments performed in triplicate are shown. RNA from non‐inoculated upper leaves of N. benthamiana and N. glutinosa accessions TW59 and TW61 agroinfected with TuYV and control, uninfiltrated plants (U) was extracted for reverse transcription‐polymerase chain reaction (RT‐PCR). ORF6 of TuYV was chosen for PCR amplification, as it is included in all viral RNAs (genomic and subgenomic). The positive controls (+) were RT‐PCRs performed on RNA extracted from the local leaves of N. benthamiana infected with TuYV. The ribulose‐1,5‐bisphosphate carboxylase/oxygenase (RuBisCO) large subunit was amplified to confirm RNA integrity. The expected size of the TuYV RT‐PCR product of 402 bp (arrowhead) was observed only in N. benthamiana, although some non‐specific products were amplified in all N. glutinosa samples, including uninfected leaves, with the large number of amplification cycles used. (B) To determine whether N. benthamiana and N. glutinosa accessions TW59 and TW61 displayed resistance against PLRV, leaves from the three plants were agroinfected with PLRV. Leaves were monitored for signs of cell death and photographed at 6 days post‐infiltration (dpi). To detect systemic spread of the virus, RNA was extracted from upper leaves of N. benthamiana and N. glutinosa accessions TW59 and TW61 agroinfected with pBIN‐PLRV. RT‐PCR targeting ORF6 of PLRV and the RuBisCO large subunit was performed on RNA from non‐inoculated upper leaves of plants infected by agroinfiltration, from control uninfected plants (U) and from infiltrated leaves of N. benthamiana (+). The expected size of the PLRV RT‐PCR product of 292 bp (arrowhead) was observed only in N. benthamiana, although some non‐specific products were amplified in all N. glutinosa samples, including uninfected leaves, with the large number of amplification cycles used.
