Figure 8.
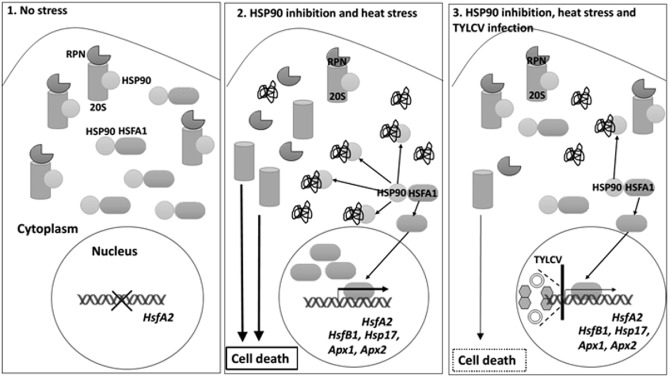
Summary of the key processes of stress response regulation by heat shock protein 90 (HSP90) in tomato plants, and down‐regulation by Tomato yellow leaf curl virus (TYLCV) infection. 1. In the absence of any stress, HSP90 binds HSFA1, one of the key regulators of the plant stress response, keeping it inactive; HSP90's substrate 26S proteasome (RPN regulatory subunits and the 20S catalytic subunit) is active. Therefore, there is no ubiquitin–proteasome system (UPS) inactivation, and consequently no cell death (CD) and no activation of HsfA2 signalling. 2. In heat‐stressed Hsp90‐silenced tomato plants, there is a massive appearance of damaged proteins, which sequester HSP90, causing the release of free HSFA1, which activates HsfA2 and downstream gene expression. The inhibition of HSP90 function also results in a dissociation of the 26S proteasome and a decrease in its peptidase activity. Inhibition of the 26S proteasome is accompanied by CD. 3. TYLCV infection (represented as virions and viral DNA) suppresses HSP90‐dependent 26S proteasome inactivation, CD and HSFA2 signal transduction pathways. Furthermore, loss of HSP90 function effects positively TYLCV accumulation: there are higher levels of viral DNA and CP, and the enrichment of CP/V2 in large aggregates, markers of a successful TYLCV infection.
