Abstract
Background:
Early life assaultive violence exposure is a potent risk factor for PTSD and other mood and anxiety disorders. Neurocircuitry models posit that increased risk is mediated by heightened emotion processing in a salience network including dorsal anterior cingulate cortex, anterior insula, and amygdala. However, the processes of reinforcement learning (RL) also engage the salience network and are implicated in responses to early life trauma and PTSD. To define their relative roles in response to early life trauma and PTSD symptoms, the current study compared engagement of the salience network during emotion processing and reinforcement learning as a function of early life assault exposure.
Methods:
Adolescent girls (n=30 physically or sexually assaulted; n = 30 healthy comparison) aged 11–17 completed two tasks during fMRI: a facial emotion processing task and RL tasks using either social or non-social stimuli. Independent component analysis was used to identify a salience network and characterize its engagement in response to emotion processing and prediction error (PE) encoding during the RL tasks.
Results:
Assault was related to greater reactivity of the salience network during emotion processing. By contrast, we found that assaulted girls demonstrated lesser encoding of negative PEs in the salience network, particularly during the social RL tasks. The dysfunction of salience network activity during emotion processing and PE encoding was not associated with PTSD symptoms.
Conclusions:
These results suggest that hyper- vs hypo-activity of the salience network among trauma-exposed youth depends on the cognitive-affective domain.
Keywords: early life trauma, PTSD, emotion, prediction errors, salience network, adolescence
Introduction
Early life assaultive violence exposure, including physical, sexual, and witnessed violence, is a potent risk factor for the development of PTSD and general psychopathology. Indeed, longitudinal studies among a nationally representative sample of adolescents have demonstrated dose-response relationships, such that prospective risk for depression, PTSD, binge drinking, cigarette smoking, and delinquent behavior increases with the severity of assaultive violence exposure(1–3). Given the potent risk for PTSD and general psychopathology associated with early life violence exposure, research has sought to identify the neural mechanisms by which this risk is conferred.
Dominant neuro circuitry models posit that the primary neural mechanisms mediating PTSD and related psychopathology following early life trauma include heightened emotion processing in the amygdala, dorsal anterior cingulate cortex (dACC), and anterior insula, with concurrent decreased emotion regulation / inhibition in medial prefrontal cortex (mPFC) and hippocampus(4–9). From a large-scale neural network perspective(10, 11), the amygdala, dACC, and anterior insula are individual nodes within a larger salience network, and hyperactivity of the individual nodes among trauma-exposed and PTSD samples has been conceptualized as reflecting hyperactivity of the larger salience network(7). While most research has focused on adult PTSD, emerging research among trauma-exposed pediatric samples suggest similar, though not identical, neural mechanisms(12), including heightened dACC during emotion processing in pediatric PTSD(13, 14), dose-response relationships between childhood trauma severity and amygdala activity during emotion processing(15), altered amygdala-insula functional connectivity following treatment in pediatric PTSD(16), and altered functional connectivity of the anterior insula and dACC in trauma-exposed youth(17, 18).
While much research has investigated the neuro circuitry of emotion and threat, the impact of early life trauma on neural mechanisms of reinforcement learning has comparatively been under-investigated(19–23). This is striking, given that traumatic stress-related psychopathology is conceptualized from a learning perspective(24–28) and a hallmark characteristic of PTSD is dysfunctional fear learning and fear extinction(4, 29, 30), processes that rely on reinforcement learning mechanisms(31–35). Existing data suggest that early life maltreatment, stress, and deprivation are associated with deficits in reinforcement learning performance (19, 20, 22) and hypo activity of lateral and medial prefrontal cortex(19). With respect to adolescents exposed to assaultive violence, our prior pilot study(36, 37) among adolescent girls during a social learning task demonstrated a linear relationship of assault exposure severity with both poorer learning performance and lesser activation in anterior insula and ACC in response to unexpected negative social outcomes, which we conceptualized as negative prediction errors. The hypoactivation of the salience network in that sample was interesting, given the typical finding of heightened salience network activity during negative emotion processing among trauma-exposed and PTSD samples. The anterior insula and dACC are widely implicated in encoding risk(38), prediction errors(39, 40), and volatility(41). As such, hypo activity during learning within these regions might be expected in trauma-exposed and PTSD samples characterized by decreased learning in response to negative prediction errors, such as during fear extinction learning. Data from this study are broadly consistent with prior reports of poorer learning among early life maltreatment samples(19, 20, 22) and negative correlations between stress exposure and dACC activation during learning(19). Nonetheless, it is relevant to mention that a recent study(23) found hyperactivity during punishment prediction errors in a more posterior mid-cingulate cortex cluster among maltreated youth that displayed elevated attention and conduct problems, but not mood or anxiety symptoms, relative to the comparison group. Accordingly, there is some inconsistency in prior studies, which might reflect differences in samples, tasks, and modeling approaches.
The purpose of the current study was to directly test the hypothesis, drawing from ours and other’s earlier results(19, 37), of differential dysfunction of the salience network during emotion processing versus reinforcement learning as a mechanism conferring risk following early life assaultive violence. We directly compared activity of the salience network (SN) during a facial emotion processing (FEP) task and during reinforcement learning (RL) tasks using either social or non-social stimuli among a sample of adolescent girls exposed to varying severities of assaultive violence. We used independent component analysis to identify a largescale salience network and computational modeling of the RL tasks to characterize prediction error signals.
Methods
Participants
Participants consisted of 60 adolescent girls, aged 11–17, enrolled at two different sites. 26 participants (n=13 exposed to assault) were recruited from Little Rock, AR and the surrounding area; 34 participants (n=17 exposed to assault) were recruited from Madison, WI and the surrounding area. This study focused on girls to reduce heterogeneity associated with sex differences in neural, hormonal, and clinical variables. Inclusion criteria for typically developing (TD) girls were absence of mental health disorders, trauma exposure, and psychiatric treatment histories. Inclusion criteria for assaulted girls were a history of directly experienced physical or sexual assault that the girl could recall. Recruitment focused on enrolling assaulted girls with a relative balance of PTSD diagnoses. Exclusion criteria for all participants included histories of psychotic symptoms, developmental disorders, neurocognitive disorders, MRI contraindications, pregnancy, history of traumatic brain injury, loss of consciousness greater than 10 min, and major medical disorders. Assaulted adolescents were not excluded based on psychotropic medication usage; however, they were required to have been stable on any medications for at least 4 weeks. Clinical and demographic characteristics are provided in Table 1. Imaging data from one participant was excluded due to excessive head motion, and imaging data from two participants were unusable due to a technical error during scanning. Behavioral analyses used all participants’ data, and the imaging analyses were based on 57 participants.
Table 1.
Clinical and demographic characteristics of the participants.
| Variable | Control | 1–2 Assaults | 3+ Assaults |
|---|---|---|---|
| N | 30 | 12 | 18 |
| Age | 15.35 (2.29) | 16.20 (1.44) | 16.26 (1.75) |
| IQ | 117.34 (21.411)a | 101.58 (18.913) | 100.61 (19.011)a |
| Ethnicity | |||
| White | 60.0% | 50.0% | 61.1% |
| Black | 20.0% | 33.3% | 11.1% |
| Asian | 3.3% | 0.0% | 0.0% |
| Hispanic, Latina | 3.3% | 8.3% | 16.7% |
| Pacific Islander | 0.0% | 0.0% | 0.0% |
| Native American | 0.0% | 0.0% | 0.0% |
| Other |
13.3% |
8.3% |
11.1% |
| Direct Assaults | .00 (.00) | 1.33 (.492)a | 4.72 (1.447)a |
| % sexual assault | 0.0% | 66.7% | 88.9% |
| % physical assault | 0.0% | 50.0% | 88.9% |
| PTSD Diagnsosis % | 0.0% | 50.0% | 55.6% |
| Mood Disorder % | 0.0% | 50.0% | 50.0% |
| Anxiety Disorder % | 0.0% | 66.7% | 72.2% |
| CAPS total severity | n/a | 39.42 (29.03) | 45.28 (30.86) |
| UCLA PTSD RI | n/a | 27.17 (19.43) | 30.72 (15.18) |
| CBCL Anxiety | 2.30 (2.25)a, b | 5.67 (6.08)a | 10.17 (5.97)b |
| CBCL Depression | 1.47 (1.89)a, b | 4.92 (3.90)a | 5.33 (2.99)b |
| Psychiatric Medications | |||
| SSRI | 0.0% | 30.8% | 38.9% |
| SNRI | 0.0% | 7.7% | 0% |
| NDRI | 0.0% | 7.7% | 0.0% |
| Mood Stabilizer | 0.0% | 7.7% | 16.7% |
| Benzo | 0.0% | 7.7% | 5.6% |
| Stimulants | 0.0% | 15.4% | 5.6% |
| Other | 0.0% | 0.0% | 5.6% |
Note. Values represent means (SDs in parentheses) or percentages. Groups with matching superscripts were significantly different (p<.05). CAPS = Clinician Administered PTSD Scale. UCLA PTSD RI = UCLA PTSD Reaction Index. CBCL =Child-behavior checklist. Mood disorder includes major depressive disorder and bipolar disorder.
Assessments
PTSD symptoms were assessed with the Clinician Administered PTSD Scale (CAPS), Child and Adolescent Version(42), and PTSD diagnoses were defined according to prior studies among youth(13, 43). Other current and lifetime mental health disorders were assessed with the MINI-KID (44). Assaultive trauma histories were characterized using the trauma assessment section of the National Survey of Adolescents (NSA) (1, 45, 46), a structured interview that uses behaviorally-specific dichotomous questions to assess seventeen categories of direct assaultive traumas across the domains of sexual assault, physical assault, and severe abuse from a caregiver. Participants also completed a corroborative assessment of childhood trauma via the Childhood Trauma Questionnaire (CTQ) (47), a widely used self-report measure assessing separate physical abuse, physical neglect, emotional abuse, emotional neglect, and sexual abuse domains of childhood maltreatment.
Reinforcement Learning Tasks
Participants completed a three-arm bandit task using either social or neutral (i.e., nonsocial or emotional stimuli) stimuli (Supplemental Figure S1). During the social task, participants were directed to select among three mock people displaying neutral facial expressions in which to invest $10, and the mock person either returned $20 or $0. The probabilities of positive returns were either 80%, 50%, or 20%, and probabilities across the mock people switched every 30 trials, for a total of 90 trials. The non-social task was structured identically, except participants selected between three houses with varying probabilities of being open (returning $20) or locked (returning $0). Participants were told their study compensation would be proportional to their performance on the task. Main trial phases of interest for this task were the decision-phase (when participants decided the person in whom to invest, duration determined by participant reaction time), the anticipation phase (while the participant was waiting for the reward outcome, jittered duration of 1.5–3s), and the feedback phase (when the participant was delivered the outcome, 2s followed by ITI of 1.5–3s). Unmodeled rest phases (fixation cross) that separated trial phases served as the baseline. More details are provided in the supplemental material.
Emotion Processing Task.
Consistent with our prior studies(15, 48), participants viewed facial stimuli and made button presses indicating decisions related to the sex of the actor. The faces contained either neutral or fearful expressions, presented either overtly (500ms) or covertly (33ms), in blocks of 10 faces. There were an equal number of female and male faces. The facial expression (neutral versus fearful) x stimulus duration (overt vs covert) factorial design constituted the primary variables of interest in this task, with rest phases (10s blocks of fixation cross) serving as the baseline. More details are provided in the supplemental material.
MRI acquisition and image preprocessing.
MRI acquisition parameters and preprocessing are described in supplemental material.
Data Analysis
Reinforcement Learning. We modeled behavior during the RL tasks using a modified version of the Rescorla-Wagner (RW) model(49, 50). This model takes the form of Vt+1=Vt+ δ * α, where V refers to expected value of a chosen action, δ is a prediction error (outcomet - Vt), and α is a learning rate that ranges from 0–1. The expected value of a chosen action changes from trial to trial based upon δ. The learning rate, α, controls the speed with which value expectations are updated, with higher learning rates leading to faster changes in expected value. We used a softmax function to transform value expectation into action probabilities through use of an exploration / exploitation parameter. Consistent with prior research(51, 52), we tested four different RW-based models that manipulated whether the model was risksensitive(53) and whether the model updated the expected value of the unchosen option(51) in a factorial design (more details provided in supplemental material). The value expectations and prediction errors of the best fitting model for the group were carried forward to the fMRI analyses using mean sample parameters(54).
Independent Component Analysis. We used Independent Component Analysis(55) (ICA) with a model order of 35 components. Task data from all runs for all participants were combined in a single ICA analysis, allowing for direct comparisons of network engagement during the RL and FEP tasks. 16 of the 35 components were deemed functional networks (versus artifact from head motion or CSF, etc.). We identified a canonical salience network consisting of peak loadings in dorsal ACC and bilateral anterior insula upon which we focused the primary analyses. Supplemental Figure S2 displays all functional networks identified.
Within-subject ICA network timecourse analyses. We characterized within-subject network encoding during the FEP and RL tasks with general linear models (GLMs) in which network timecourses were regressed onto task-specific design matrices that were created in AFNI(56). For the FEP, the design matrix consisted of four regressors for each of the task conditions (i.e., fear vs neutral x overt vs covert factorial design). For the RL tasks, the design matrices consisted of regressors for the outcome, anticipation, and decision phases for the tasks. The outcome phase was parametrically modulated by signed prediction errors (PEs) and the anticipation and decision phases were parametrically modulated by V(52, 54, 57, 58). Using Matlab, we regressed the network timecourses onto these design matrices to estimate β coefficients that were carried forward into second-level analyses. Given that reward outcomes are highly collinear with signed PEs(59) (i.e., negative PEs only occur on loss trials), we used a model comparison approach to demonstrate that salience network activity better reflects PE encoding rather than reward outcome processing (supplemental material).
Between-subject ICA network analyses. The primary analyses focused on testing associations with assault exposure severity and component encoding during the FEP and RL tasks using linear mixed-effects (LME) models (Matlab’s fitlme.m). We hypothesized a positive association between assault severity and SN activity during FEP and a negative association between assault severity and SN encoding of negative PEs. Consistent with a dose-response relationship between assault exposure severity and risk for psychopathology(1, 60), and identical to our prior study among a separate sample(37), we tested a linear effect of assault exposure by coding an ordinal variable with three levels: TD (coded = 0, n = 30), girls exposed to 1–2 categories of assaults (coded = 1; n = 12), and girls exposed to 3 or more categories of assaults (coded = 2; n = 18). This resulted in assault groups that were matched in PTSD diagnoses, caregiver-rated anxiety and depression, and psychotropic medication use (Table 1). An identical LME was then conducted with the CTQ. Secondary analyses focused on testing the impact of current PTSD symptom severity (CAPS total severity scores) on variables of interest among the assaulted girls (n=30). All models included fixed effects of ordinal assault exposure severity (or PTSD symptom severity), age, verbal IQ, and scanning site, and random effects for task contrast and head motion (framewise displacement; FD) nested within a subject factor. We controlled for alpha inflation due to multiple comparisons across the 16 identified ICA networks with Bonferroni correction.
Results
Behavioral Performance on RL tasks.
The risk-sensitive anti-correlated RW model (supplemental material) provided best fit to the behavioral data in both groups (Figure 1A), and choice behavior was well-described by this model (Figure 1B). There was no overall difference in performance between the social and non-social task (p=.20; Supplemental Figure S2). Mixed effect models were non-significant for relationships between correct responses (p=.09) and soft max betas (p=.068) as a function of assault exposure severity (Figure 1C).
Figure 1.
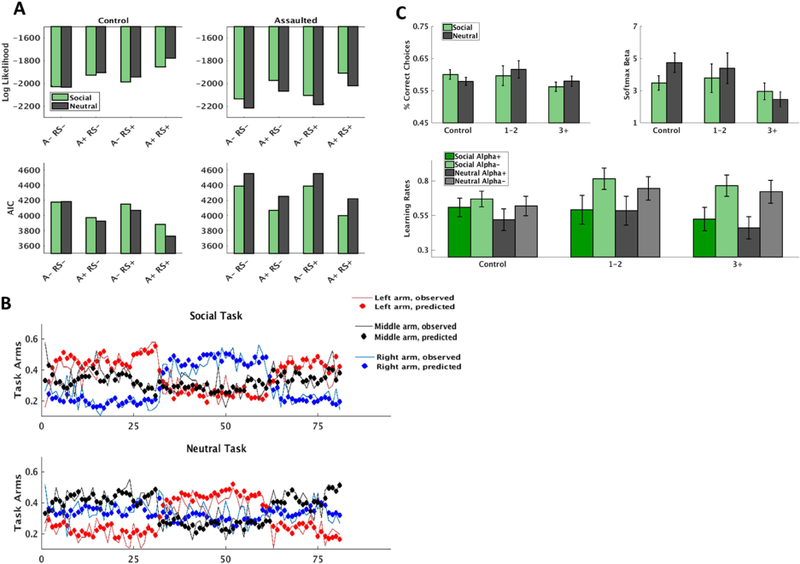
Modeling of the reinforcement learning (RL) tasks. A) Four variations of the RescorlaWagner model was tested: not anti-correlated or risk-sensitive (Anti- RS-), not anti-correlated and risk-sensitive (Anti- RS+), anti-correlated and not risk-sensitive (Anti+ RS-), and anticorrelated and risk-sensitive (Anti+ RS+). The Anti+ RS+ was the best fitting model in both groups according to both log-likelihood and Akaike Information Criterion (AIC). B) Mean proportion of model predicted choices and observed choices across participants for each of the three arms of the social and non-social reinforcement learning tasks. Also evident is the change in reward structure every 30 trials. C) Comparison of correct responses (percent of reinforced trials), softmax exploration/exploitation parameters, and learning rates (learning rates for both positive prediction errors and negative prediction errors, in accordance with the Anti+ RS+ model) as a function of early life assaultive violence exposure severity. ‘1–2’ indicates girls exposed to 1–2 categories of assault exposure (n=12); ‘3+’ indicates girls exposed to 3 or more categories of assault exposure (n=18).
Differential Salience Network Activity as a Function of Assault Exposure Severity.
We first tested the higher-order assault exposure severity x task (RL versus FEP task) interaction and initially compared PE encoding during both RL tasks to facial emotion processing (FEP) collapsed across stimulus category and observed a significant assault x task interaction, t(327)=3.56, p < .001 (Bonferroni corrected p = .007). This higher-order interaction was then decomposed by testing separate models for the RL and FEP tasks.
For the RL tasks, we observed a main effect of assault exposure severity indicating weakened SN encoding of negative PEs, t(99)=−3.50, p < .001, that was not moderated by social versus non-social RL task, t(99)=1.16, p=.25. However, the relationship with assault exposure severity was more robust during the social, t(48)=−3.86, p < .001, compared to the non-social, t(47)=−1.74, p=.09, RL task (Figure 2A). The relationship between weakened SN encoding of negative prediction errors and assault exposure severity remained when including PTSD symptom severity as a covariate, t(98)=−3.11, p=.002.
Figure 2.
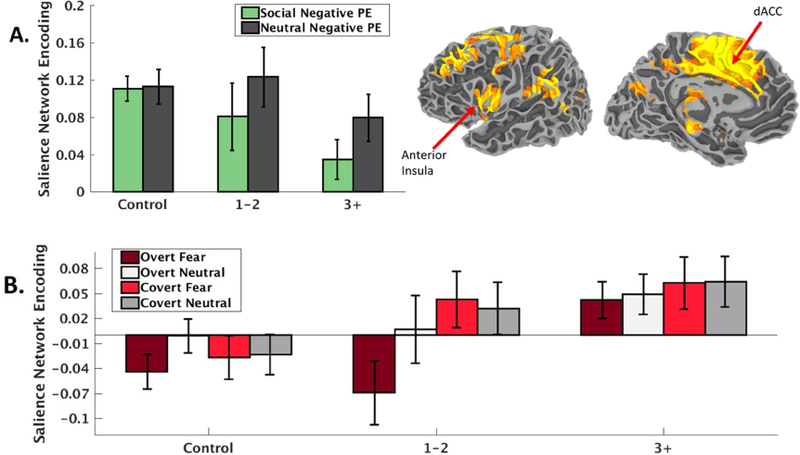
A) Salience network (depicted top right, radiological convention) encoding (regression coefficients from the first-level ICA timecourse modeling) of negative prediction errors during the reinforcement learning task decreases with the severity of early life assaultive violence exposure. B) Salience network activity (regression coefficients from the first-level ICA timecourse modeling) during facial emotion processing increases with the severity of early life assaultive violence exposure. ‘1–2’ indicates girls exposed to 1–2 categories of assault exposure (n=12); ‘3+’ indicates girls exposed to 3 or more categories of assault exposure (n=18). dACC = Dorsal anterior cingulate cortex. PE = prediction error.
For the FEP task, while the overall main effect of assault exposure severity on FEP was not significant, t(216)=1.87, p=.063, there was a group x facial expression (neutral vs fear) x duration (covert vs overt) interaction, t(212)=2.57, p=.011. This interaction was attributed to greater SN responses to overt fear faces in the highly assaulted compared to both other groups, t(106)=2.09, p=.039 (Figure 2B). The group x facial expression x duration interaction remained significant when controlling for CAPS PTSD symptom severity, t(211)=2.57, p=.011. There was no relationship between SN encoding of negative PEs during the RL tasks and SN activity during FEP nor an interaction with assault exposure severity (all ps > .34), supporting the two processes as dissociable responses of the SN to adolescent assault exposure.
Differential Salience Network Activity with a Corroborative Measure of Early Life Trauma.
We repeated analyses of SN task engagement using the CTQ total score as a corroborative measure of early life maltreatment severity. These analyses also demonstrated weakened SN encoding of negative PEs as a function of greater CTQ total scores, t(99)=−2.27, p=.026 (Figure 3A), and no interaction with social versus non-social RL task, t(99)=1.08, p = .28. For the FEP task, this analysis supported a main effect of greater CTQ total scores on SN responses during facial emotion processing, t(216)=2.18, p=.03 (Figure 3B), and a non-significant CTQ x task interaction, t(216)=−1.87, p=.06.
Figure 3.
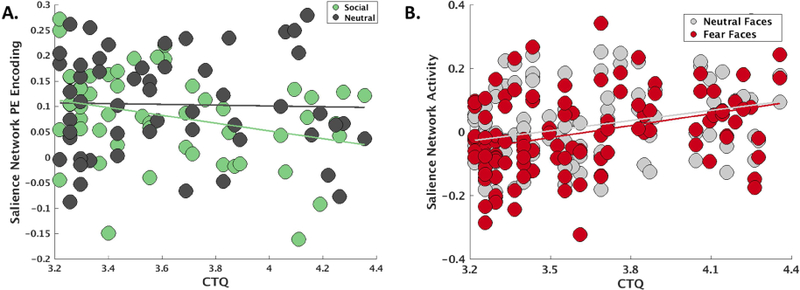
A) Salience network encoding (regression coefficients from the first-level ICA timecourse modeling) of negative prediction errors during the reinforcement learning task also decreased with the severity of early life trauma as measured by the physical, sexual, and emotional abuse subscales of the Childhood Trauma Questionnaire (CTQ), particularly for social stimuli. B) Salience network activity (regression coefficients from the first-level ICA timecourse modeling) during facial emotion processing increased as a function of early life trauma on the CTQ, particularly for fear faces. PE = prediction error.
Exploratory Analyses of Network Engagement during Facial Emotion Processing.
Given that we observed primarily SN deactivation in the control group during the FEP task, we hypothesized that perhaps the decreased SN engagement in the control group compared to relative increase in SN engagement in severely assaulted girls was due to differences in alternative network engagement patterns between the groups. Other than a motor network, which was robustly activated in each group due to the considerable motor demands of the block design task (Supplemental Tables S1-S2), the network that was most strongly activated in the control group, t(27)=9.06, p<.001 (Bonferroni corrected p < .001), was a network with dominant loadings in bilateral fusiform gyrus, caudate, and nucleus accumbens (Fig 4.) subsequently referred to as a fusiform-striatum (FS) network and attributed to object and reward processing(61–63). Further demonstrating the functional relevance of the FS network, this was also the most robustly engaged network during positive PE encoding in the control group, t(27)=10.21, p<.001 (Bonferroni corrected p < .001; Supplemental Tables S1-S2). Given the robust engagement of the FS network during FEP in the control group, we then tested the hypothesis of opposing patterns of network engagement during FEP between the control and assaulted groups and observed a significant assault exposure severity x network (SN vs FS) interaction, t(436)=2.81, p = .005, such that SN activity during FEP increased linearly across assault exposures (Figure 2B), while FS network activity decreased linearly across assault exposure, t(216)=−2.13, p=.034 (Figure 4A). Functionally opposing responses of the SN and FS during FEP across participants was further supported by a model result demonstrating a strong negative relationship between SN and FS recruitment during FEP, t(225)=−4.53, p<.001 (Figure 4B).
Figure 4.
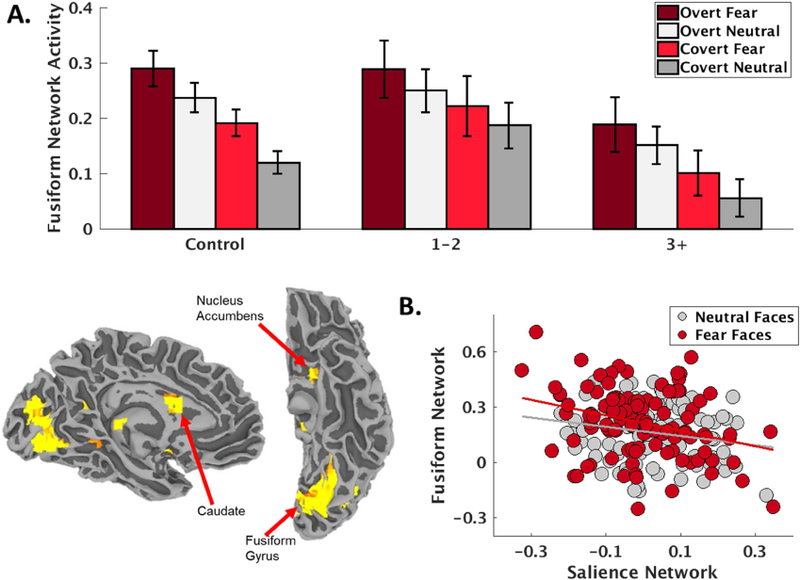
A) A fusiform-striatum network (depicted bottom left, radiological convention) was robustly engaged during facial emotion processing in the control girls, and engagement of this network decreased with the severity of early life assaultive violence exposure. B) There was a strong negative relationship between activation (regression coefficients from the first-level ICA timecourse modeling) of the fusiform-striatum and salience networks during facial emotion processing, particularly for fear faces.
Network Response to PE and FEP as a Function of PTSD Symptom Severity.
When examining the effect of PTSD symptom severity among the assaulted girls, the higher-order group x task interaction comparing both RL tasks to facial emotion processing (FEP) collapsed across stimulus category did not reveal any significant interaction or main effects of PTSD (all ps > .6).
Ruling out confounds related to medication usage and scanning site.
Supplemental material describes additional analyses demonstrating the above results are not confounded by psychotropic medication use or differences in scanning sites.
Discussion
Consistent with dominant models(7, 64), the results demonstrated that SN activity during emotion processing increased with the severity of assaultive violence exposure. By contrast, consistent with our pilot study(37) and previous reports of altered reinforcement learning mechanisms among maltreated youth(19, 20, 23), SN encoding of negative prediction errors decreased with the severity of assault exposure. These effects were observed when examining the severity of assaultive violence exposure and also when examining severity of early life maltreatment as measured by the CTQ. This differential response of the SN has implications for our understanding of the neural mechanisms by which early life trauma confers risk for PTSD and other mood and anxiety disorders.
The consistent observation of SN hyperactivity towards threat provides a powerful explanation for many clinical symptoms among early trauma victims that develop PTSD and other mood and anxiety disorders, including attentional biases towards threat(65, 66), increased fear learning(67, 68), and increased startle responses(69, 70). Here, we observed increased SN activity for both neutral and fearful faces in the highly assaulted group, consistent with other reports among trauma-exposed samples(71–73), and suggests either heightened salience processing of facial stimuli per se or generalized threat responses towards neutral facial expressions. While these results are consistent with prior neurocircuitry models of early life trauma, prior models and data have not suggested clear hypotheses regarding SN encoding of negative prediction errors among trauma-exposed youth. One possibility would have been to expect SN hyperactivity among trauma-exposed youth across all cognitive domains. The currents results are incompatible with this possibility, and instead suggest that altered SN responses among early life trauma victims depends on the specific cognitive-affective domain. In our control sample we observed robust negative PE encoding in the salience network, suggesting that negative PEs normatively function as salient signals. Nonetheless, early life trauma was associated with weakened SN encoding of negative PEs. By contrast, during facial emotion processing, the control group did not reliably recruit the SN and an exploratory analysis suggested that facial emotion processing was instead mostly strongly associated with engagement of a separate fusiform-striatum (FS) network consistent with object recognition and reward processing(61–63). Among assaulted girls, there was evidence for strengthened engagement of the SN, particularly for overt fear faces, and weakened engagement of the FS network in an exploratory analysis. Consistent with opposing recruitment of the SN versus FS network during facial emotion processing, we observed a strong negative relationship between SN activity and FS network activity during this task. These data suggest qualitatively different network activation profiles to facial emotion versus negative PE signals among typically developing and assault-exposed youth, with possible reciprocal roles for the SN and FS networks in normative FEP.
One explanation of the opposing roles of the SN among trauma-exposed youth depending on cognitive domain might be that prolonged SN hyperactivity to threat results in subsequent blunting of SN response to other signals. If this were the case, then one would expect that SN hyperactivity to threat would be negatively related to SN hypoactivity to negative PEs. The current data failed to identify a significant relationship between SN activation during FEP and SN encoding of negative prediction errors, and thus are incompatible with this possibility. Instead, the data suggest that SN hyperactivity in a particular cognitive-affective domain among early life trauma victims (e.g., facial emotion processing) should not necessarily be expected to generalize to other cognitive-affect domains (e.g., negative PE encoding), which is consistent with dissociable mechanisms in the SN and distinct patterns of SN alterations among trauma-exposed youth. This pattern of data suggests the utility of SN models of early life trauma exposure may come from further careful delineation of the SN activity across clinicallyrelevant cognitive domains.
In much the same way that SN hyperactivity towards threat cues among trauma victims implies an adaptive response following trauma exposure, it may be the case that SN hypoactivity to negative PEs similarly serves an adaptive purpose. Whereas the SN and its primary nodes, the dACC and anterior insula, are widely implicated in attention, awareness, and cognitive control(10, 11, 74–76), the dACC and anterior insula are also widely implicated in encoding computational mechanisms of RL, including risk, prediction errors, uncertainty, and exploration/foraging (38–40, 49, 77–79). Indeed, negative PEs (e.g., omission of an expected outcome) are primary teaching signals for extinction, and PTSD is widely characterized by poorer fear extinction learning(80) and recall(29, 30). Accordingly, weakened SN encoding of negative PEs might reflect a compensatory mechanism that promotes retention of learned associations, which might have an adaptive purpose in dangerous environments (e.g., abusive early social environments). Relatedly, a recent study found hyperactive encoding of stimulus associability (i.e., similar to a dynamic learning rate(35, 81)), but not prediction errors, in the anterior insula and amygdala among combat veterans with PTSD(82), consistent with a dissociation in SN hyperactivity for maintaining attentional vigilance versus computing discrepancies with prior expectations. Additionally, while we did not observe specificity for weakened encoding of social versus non-social negative PEs, the overall pattern of results suggested more robust impairments of PE encoding for social stimuli. In this sample of youth who directly experienced violence inflicted upon them by another person, weakened SN responsivity towards unexpectedly negative social behavior could reflect learned blunted responding that develops as an adaption following toxic early social environments and helps maintain learned social associations. Weakened anterior insula has also been linked to decreased detection of untrustworthiness(83) and thereby possibly suggests a mechanism explaining heightened risk of revictimization among youth exposed to early life trauma(2, 84). Finally, the relationships observed between SN encoding of negative PEs and facial emotion processing were most strongly related to early life trauma severity (i.e., both assault exposure severity and the CTQ), remained when controlling for PTSD symptom severity, and did not scale with PTSD symptom severity among the assaulted adolescents. This pattern of results suggests that altered SN responses best reflects a risk factor for PTSD, rather than a marker of PTSD itself.
The current study is not without limitations. First, the study is limited to adolescent girls and generalization to boys is not warranted. Second, while effects remained consistent at both sites, it is possible that heterogeneity due to site differences obscured detection of other prominent effects. Third, the sample was heterogenous with respect to comorbidity and medication use, which reflects real-world clinical samples but may limit specificity of inferences. Fourth, the current sample was selected based on interpersonal traumas and it is not clear whether similar results would be expected among samples exposed to non-interpersonal traumas. Fifth, the analyses regarding the FS network and alternative network engagement in control versus assaulted youth were exploratory, and future research is needed to continue investigating patterns of alternative and opposing network activation among healthy and traumaexposed youth.
Supplementary Material
Funding acknowledgement
Portions of this research were funded by MH106860, MH108753, MH100267, and the Brain and Behavior Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Cisler JM, Begle AM, Amstadter AB, Resnick HS, Danielson CK, Saunders BE, Kilpatrick DG (2012): Exposure to interpersonal violence and risk for PTSD, depression, delinquency, and binge drinking among adolescents: Data from the NSA-R. J Trauma Stress 25: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cisler JM, Amstadter AB, Begle AM, Resnick HS, Danielson CK, Saunders BE, Kilpatrick DG (2011): PTSD symptoms, potentially traumatic event exposure, and binge drinking: A prospective study with a national sample of adolescents. J Anxiety Disord 25: 978–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cisler JM, Amstadter AB, Begle AM, Resnick HS, Danielson CK, Saunders BE, Kilpatrick DG (2011): A prospective examination of the relationships between PTSD, exposure to assaultive violence, and cigarette smoking among a national sample of adolescents. Addict Behav 36: 994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rauch SL, Shin LM, Phelps EA (2006): Neurocircuitry Models of Posttraumatic Stress Disorder and Extinction: Human Neuroimaging Research—Past, Present, and Future. Biol Psychiatry 60: 376–382. [DOI] [PubMed] [Google Scholar]
- 5.Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, et al. (2012): Biological studies of post-traumatic stress disorder. Nat Rev Neurosci 13: 769–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Admon R, Milad MR, Hendler T (2013): A causal model of post-traumatic stress disorder disentangling predisposed from acquired neural abnormalities. Trends Cogn Sci 17: 337–347. [DOI] [PubMed] [Google Scholar]
- 7.Patel R, Spreng RN, Shin LM, Girard TA (2012): Neurocircuitry models of posttraumatic stress disorder and beyond: A meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev 36: 2130–2142. [DOI] [PubMed] [Google Scholar]
- 8.Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, et al. (2012): Limbic Scars: Long-Term Consequences of Childhood Maltreatment Revealed by Functional and Structural Magnetic Resonance Imaging. Biol Psychiatry 71: 286– 293. [DOI] [PubMed] [Google Scholar]
- 9.Teicher MH, Samson JA (2013): Childhood Maltreatment and Psychopathology: A Case for Ecophenotypic Variants as Clinically and Neurobiologically Distinct Subtypes. Am J Psychiatry 170: 1114–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menon V (2011): Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 15: 483–506. [DOI] [PubMed] [Google Scholar]
- 11.Bressler SL, Menon V (2010): Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci 14: 277–290. [DOI] [PubMed] [Google Scholar]
- 12.Herringa RJ (2017): Trauma, PTSD, and the Developing Brain. Curr Psychiatry Rep 19: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolf RC, Herringa RJ (2016): Prefrontal–Amygdala Dysregulation to Threat in Pediatric Posttraumatic Stress Disorder. Neuropsychopharmacology 41: 822–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keding TJ, Herringa RJ (2016): Paradoxical Prefrontal-Amygdala Recruitment to Angry and Happy Expressions in Pediatric Posttraumatic Stress Disorder. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 41: 2903–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cisler JM, Privratsky A, Smitherman S, Herringa RJ, Kilts CD (2018): Large-scale brain organization during facial emotion processing as a function of early life trauma among adolescent girls. NeuroImage Clin 17: 778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cisler JM, Sigel BA, Steele JS, Smitherman S, Vanderzee K, Pemberton J, et al. (2016): Changes in functional connectivity of the amygdala during cognitive reappraisal predict symptom reduction during trauma-focused cognitive-behavioral therapy among adolescent girls with post-traumatic stress disorder. Psychol Med 1–11. [DOI] [PubMed]
- 17.Marusak HA, Etkin A, Thomason ME (2015): Disrupted insula-based neural circuit organization and conflict interference in trauma-exposed youth. Neuro Image Clin 8: 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cisler JM, Scott Steele J, Smitherman S, Lenow JK, Kilts CD (2013): Neural processing correlates of assaultive violence exposure and PTSD symptoms during implicit threat processing: a network-level analysis among adolescent girls. Psychiatry Res 214: 238– 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harms MB, Shannon Bowen KE, Hanson JL, Pollak SD (2017): Instrumental learning and cognitive flexibility processes are impaired in children exposed to early life stress. Dev Sci [DOI] [PMC free article] [PubMed]
- 20.Hanson JL, van den Bos W, Roeber BJ, Rudolph KD, Davidson RJ, Pollak SD (2017): Early adversity and learning: implications for typical and atypical behavioral development. J Child Psychol Psychiatry 58: 770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dennison MJ, Rosen ML, Sambrook KA, Jenness JL, Sheridan MA, McLaughlin KA (2017): Differential Associations of Distinct Forms of Childhood Adversity With Neurobehavioral Measures of Reward Processing: A Developmental Pathway to Depression. Child Dev [DOI] [PMC free article] [PubMed]
- 22.Sheridan MA, McLaughlin KA, Winter W, Fox N, Zeanah C, Nelson CA (2018): Early deprivation disruption of associative learning is a developmental pathway to depression and social problems. Nat Commun 9: 2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerin MI, Puetz VB, Blair RJR, White S, Sethi A, Hoffmann F, et al. (2017): A neurocomputational investigation of reinforcement-based decision making as a candidate latent vulnerability mechanism in maltreated children. Dev Psychopathol 29: 1689–1705. [DOI] [PubMed] [Google Scholar]
- 24.Rothbaum BO, Davis M (2003): Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci 1008: 112–121. [DOI] [PubMed] [Google Scholar]
- 25.Yehuda R, LeDoux J (2007): Response Variation following Trauma: A Translational Neuroscience Approach to Understanding PTSD. Neuron 56: 19–32. [DOI] [PubMed] [Google Scholar]
- 26.Lissek S, van Meurs B (2015): Learning Models of PTSD: Theoretical Accounts and Psychobiological Evidence. Int J Psychophysiol Off J Int Organ Psychophysiol 98: 594– 605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perusini JN, Meyer EM, Long VA, Rau V, Nocera N, Avershal J, et al. (2016): Induction and Expression of Fear Sensitization Caused by Acute Traumatic Stress. Neuro psycho pharmacol Off Publ Am Coll Neuro psycho pharmacol 41: 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rau V, DeCola JP, Fanselow MS (2005): Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev 29: 1207–1223. [DOI] [PubMed] [Google Scholar]
- 29.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. (2009): Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 66: 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garfinkel SN, Abelson JL, King AP, Sripada RK, Wang X, Gaines LM, Liberzon I (2014): Impaired contextual modulation of memories in PTSD: an fMRI and psychophysiological study of extinction retention and fear renewal. J Neurosci Off J Soc Neurosci 34: 13435–13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gershman SJ, Niv Y (2012): Exploring a latent cause theory of classical conditioning. Learn Behav 40: 255–268. [DOI] [PubMed] [Google Scholar]
- 32.Gershman SJ, Hartley CA (2015): Individual differences in learning predict the return of fear. Learn Behav 43: 243–250. [DOI] [PubMed] [Google Scholar]
- 33.Redish AD, Jensen S, Johnson A, Kurth-Nelson Z (2007): Reconciling reinforcement learning models with behavioral extinction and renewal: implications for addiction, relapse, and problem gambling. Psychol Rev 114: 784. [DOI] [PubMed] [Google Scholar]
- 34.Delgado MR, Li J, Schiller D, Phelps EA (2008): The role of the striatum in aversive learning and aversive prediction errors. Philos Trans R Soc B Biol Sci 363: 3787–3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Schiller D, Schoenbaum G, Phelps EA, Daw ND (2011): Differential roles of human striatum and amygdala in associative learning. Nat Neurosci 14: 1250–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lenow J, Cisler J, Bush K (2015): Altered Trust Learning Mechanisms Among Female Adolescent Victims of Interpersonal Violence. J Interpers Violence 10.1177/0886260515604411. [DOI] [PubMed]
- 37.Lenow JK, Scott Steele J, Smitherman S, Kilts CD, Cisler JM (2014): Attenuated behavioral and brain responses to trust violations among assaulted adolescent girls. Psychiatry Res 223: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Preuschoff K, Quartz SR, Bossaerts P (2008): Human insula activation reflects risk prediction errors as well as risk. J Neurosci 28: 2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown JW, Braver TS (2005): Learned predictions of error likelihood in the anterior cingulate cortex. Science 307: 1118–1121. [DOI] [PubMed] [Google Scholar]
- 40.Alexander WH, Brown JW (2011): Medial prefrontal cortex as an action-outcome predictor. Nat Neurosci 14: 1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Behrens TEJ, Woolrich MW, Walton ME, Rushworth MFS (2007): Learning the value of information in an uncertain world. Nat Neurosci 10: 1214–1221. [DOI] [PubMed] [Google Scholar]
- 42.Nader K, Blake D, Pynoos RS, Newman E, Weathers F (n.d.): Clinician-Administered PTSD Scale, Child and Adolescent Version White River Junction, VT: National Center for PTSD. [Google Scholar]
- 43.Cohen JA, Deblinger E, Mannarino AP, Steer RA (2004): A multisite, randomized controlled trial for children with sexual abuse–related PTSD symptoms. J Am Acad Child Adolesc Psychiatry 43: 393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheehan DV, Sheehan KH, Shytle RD, Janavs J, Bannon Y, Rogers JE, et al. (2010): Reliability and Validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID). J Clin Psychiatry 71: 313–326. [DOI] [PubMed] [Google Scholar]
- 45.Kilpatrick DG, Acierno R, Saunders B, Resnick HS, Best CL, Schnurr PP (2000): Risk factors for adolescent substance abuse and dependence: Data from a national sample. J Consult Clin Psychol 68: 19–30. [DOI] [PubMed] [Google Scholar]
- 46.Kilpatrick DG, Ruggiero KJ, Acierno R, Saunders BE, Resnick HS, Best CL (2003): Violence and risk of PTSD, major depression, substance abuse/dependence, and comorbidity: Results from the National Survey of Adolescents. J Consult Clin Psychol 71: 692–700. [DOI] [PubMed] [Google Scholar]
- 47.Bernstein DP, Fink L (1998): Childhood trauma questionnaire: A retrospective self-report: Manual Harcourt Brace & Company. [Google Scholar]
- 48.Cisler JM, Sigel BA, Kramer TL, Smitherman S, Vanderzee K, Pemberton J, Kilts CD (2015): Amygdala response predicts trajectory of symptom reduction during TraumaFocused Cognitive-Behavioral Therapy among adolescent girls with PTSD. J Psychiatr Res 71: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rushworth MFS, Behrens TEJ (2008): Choice, uncertainty and value in prefrontal and cingulate cortex. Nat Neurosci 11: 389–397. [DOI] [PubMed] [Google Scholar]
- 50.Sutton RS, Barto AG (1998): Learning: An Introduction Cambridge, MA: MIT Press. [Google Scholar]
- 51.Hauser TU, Iannaccone R, Walitza S, Brandeis D, Brem S (2015): Cognitive flexibility in adolescence: Neural and behavioral mechanisms of reward prediction error processing in adaptive decision making during development. NeuroImage 104: 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ross MC, Lenow JK, Kilts CD, Cisler JM (2018): Altered neural encoding of prediction errors in assault-related posttraumatic stress disorder. J Psychiatr Res 103: 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niv Y, Edlund JA, Dayan P, O’Doherty JP (2012): Neural Prediction Errors Reveal a RiskSensitive Reinforcement-Learning Process in the Human Brain. J Neurosci 32: 551– 562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daw ND, O’Doherty JP, Dayan P, Seymour B, Dolan RJ (2006): Cortical substrates for exploratory decisions in humans. Nature 441: 876–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calhoun VD, Adali T, Pearlson GD, Pekar JJ (2001): A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 14 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cox RW (1996): AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res Int J 29: 162–173. [DOI] [PubMed] [Google Scholar]
- 57.Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD (2006): Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature 442: 1042– 1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Skvortsova V, Palminteri S, Pessiglione M (2014): Learning To Minimize Efforts versus Maximizing Rewards: Computational Principles and Neural Correlates. J Neurosci 34: 15621–15630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Erdeniz B, Rohe T, Done J, Seidler R (2013): A simple solution for model comparison in bold imaging: the special case of reward prediction error and reward outcomes. Front Neurosci 7. doi: 10.3389/fnins.2013.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Neuner F, Schauer M, Karunakara U, Klaschik C, Robert C, Elbert T (2004): Psychological trauma and evidence for enhanced vulnerability for posttraumatic stress disorder through previous trauma among West Nile refugees. BMC Psychiatry 4: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Knutson B, Adams CM, Fong GW, Hommer D (2001): Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 21: RC159–RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Balleine BW, Delgado MR, Hikosaka O (2007): The role of the dorsal striatum in reward and decision-making. J Neurosci 27: 8161–8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bar M, Tootell RB, Schacter DL, Greve DN, Fischl B, Mendola JD, et al. (2001): Cortical mechanisms specific to explicit visual object recognition. Neuron 29: 529–535. [DOI] [PubMed] [Google Scholar]
- 64.Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, et al. (2012): Biological studies of post-traumatic stress disorder. Nat Rev Neurosci 13: 769–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH (2007): Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull 133: 1–24. [DOI] [PubMed] [Google Scholar]
- 66.Cisler JM, Koster EHW (2010): Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clin Psychol Rev 30: 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wessa M, Flor H (2007): Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. Am J Psychiatry 164: 1684–1692. [DOI] [PubMed] [Google Scholar]
- 68.Acheson DT, Geyer MA, Baker DG, Nievergelt CM, Yurgil K, Risbrough VB, MRS-II Team (2015): Conditioned fear and extinction learning performance and its association with psychiatric symptoms in active duty Marines. Psychoneuroendocrinology 51: 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pole N (2007): The psychophysiology of posttraumatic stress disorder: a meta-analysis. Psychol Bull 133: 725–746. [DOI] [PubMed] [Google Scholar]
- 70.Orr SP, Lasko NB, Shalev AY, Pitman RK (1995): Physiologic responses to loud tones in Vietnam veterans with posttraumatic stress disorder. J Abnorm Psychol 104: 75–82. [DOI] [PubMed] [Google Scholar]
- 71.Garrett AS, Carrion V, Kletter H, Karchemskiy A, Weems CF, Reiss A (2012): Brain activation to facial expressions in youth with PTSD symptoms. Depress Anxiety 29: 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hendler T, Rotshtein P, Yeshurun Y, Weizmann T, Kahn I, Ben-Bashat D, et al. (2003): Sensing the invisible: differential sensitivity of visual cortex and amygdala to traumatic context. Neuroimage 19: 587–600. [DOI] [PubMed] [Google Scholar]
- 73.Brunetti M, Sepede G, Mingoia G, Catani C, Ferretti A, Merla A, et al. (2010): Elevated response of human amygdala to neutral stimuli in mild posttraumatic stress disorder: neural correlates of generalized emotional response. Neuroscience 168: 670–679. [DOI] [PubMed] [Google Scholar]
- 74.Menon V, Uddin LQ (2010): Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214: 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ (2011): The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci 12: 154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Craig ADB (2009): How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci 10: 59–70. [DOI] [PubMed] [Google Scholar]
- 77.Blanchard TC, Gershman SJ (2018): Pure correlates of exploration and exploitation in the human brain. Cogn Affect Behav Neurosci 18: 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shenhav A, Straccia MA, Botvinick MM, Cohen JD (2016): Dorsal anterior cingulate and ventromedial prefrontal cortex have inverse roles in both foraging and economic choice. Cogn Affect Behav Neurosci 16: 1127–1139. [DOI] [PubMed] [Google Scholar]
- 79.Kolling N, Behrens TEJ, Mars RB, Rushworth MFS (2012): Neural mechanisms of foraging. Science 336: 95–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Duits P, Cath DC, Lissek S, Hox JJ, Hamm AO, Engelhard IM, et al. (2015): Updated Meta-Analysis of Classical Fear Conditioning in the Anxiety Disorders. Depress Anxiety 32: 239–253. [DOI] [PubMed] [Google Scholar]
- 81.Le Pelley ME (2004): The role of associative history in models of associative learning: A selective review and a hybrid model. Q J Exp Psychol Sect B 57: 193–243. [DOI] [PubMed] [Google Scholar]
- 82.Brown VM, Zhu L, Wang JM, Frueh BC, King-Casas B, Chiu PH (2018): Associabilitymodulated loss learning is increased in posttraumatic stress disorder. eLife 7: e30150 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Castle E, Eisenberger NI, Seeman TE, Moons WG, Boggero IA, Grinblatt MS, Taylor SE (2012): Neural and behavioral bases of age differences in perceptions of trust. Proc Natl Acad Sci U S A 109: 20848–20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Messman-Moore TL, Brown AL (2006): Risk perception, rape, and sexual revictimization: A prospective study of college women. Psychol Women Q 30: 159–172. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


