Figure 2.
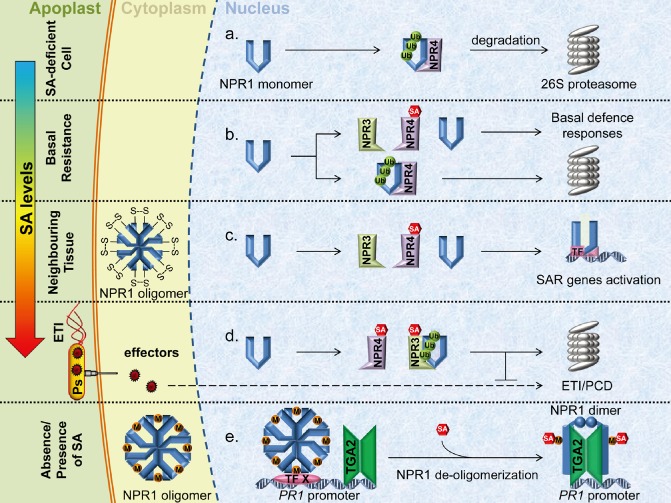
Models for salicylic acid (SA) perception in planta. (a–d) The data of Fu et al. (2012) indicate that NON‐EXPRESSOR OF PATHOGENESIS‐RELATED 3 (NPR3) and NPR4 function as SA receptors. (a) Binding of NPR1 by NPR4 in the absence of SA leads to NPR1 degradation via the 26S proteasome. The Cullin3 (CUL3) adaptor protein is omitted for simplicity. (b) Basal SA levels allow for binding of SA to NPR4, thereby limiting the ability of NPR4 to act as a CUL3 substrate adaptor and binding NPR1 for degradation. Low levels of NPR1 accumulate and subsequently activate basal resistance responses, whilst some NPR4‐dependent NPR1 degradation continues. (c) Moderate SA levels experienced in effector‐triggered immunity (ETI) in neighbouring cells (systemic tissue) allow for SA binding to NPR4, limit NPR4–NPR1 interaction and, in turn, permit NPR1‐dependent expression of systemic acquired resistance (SAR) genes. A pool of NPR1 undergoes degradation via NPR3 interaction. (d) Cells subjected to direct avirulent pathogen attack experience high SA accumulation, leading to subsequent NPR3‐dependent NPR1 degradation and ETI/programmed cell death (PCD) inhibition. (e) Wu et al. (2012) postulate that NPR1 functions as the SA receptor. The NPR1 oligomer contains transitional metal ions (M), such as copper, to facilitate the binding of SA. Reducing conditions in the cell begin the de‐oligomerization of NPR1, but SA is required for complete oligomer disassembly. The nuclear NPR1 oligomer interacts with the PATHOGENESIS‐RELATED 1 (PR1) promoter via an unknown transcription factor (TF X) and binds with a TGA2 dimer on SA induction.
