Figure 6.
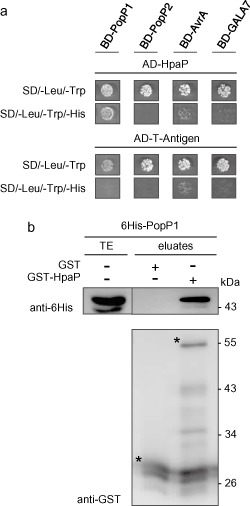
HpaP interacts specifically with the type III effector (T3E) PopP1. (a) Interaction studies between HpaP and several T3Es. Yeast cells were co‐transformed by AD‐HpaP and BD‐PopP1, BD‐PopP2, BD‐AvrA or BD‐GALA7. Double transformation and interaction were tested by plating yeasts on synthetic dropout medium lacking leucine and tryptophan (SD/–Leu/–Trp) and synthetic dropout medium lacking leucine, tryptophan and histidine (SD/–Leu/–Trp/–His), respectively. 3‐Aminotriazole (3‐AT) was added to suppress autoactivation when necessary. Three biological replicates were performed giving the same results. (b) In vitro validation of the interaction between HpaP and PopP1. Glutathione S‐transferase (GST) and GST‐HpaP were immobilized on glutathione sepharose and incubated with an Escherichia coli lysate containing 6His‐PopP1. Total cell lysates (TE) and eluted proteins (eluates) were analysed using antibodies directed against GST and the 6His epitope. Bands corresponding to GST and GST fusion proteins are marked by asterisks; lower bands represent degradation products. Experiments were repeated twice with similar results. AD, activation domain; BD, binding domain; His, histidine.
