SUMMARY
Taxonomic relationships: Cucumber mosaic virus (CMV) is the type species of the genus Cucumovirus in the family Bromoviridae, which also encompasses the Peanut stunt virus (PSV) and the Tomato aspermy virus (TAV). Nucleotide sequence similarity among these three cucumoviruses is 60%–65%. CMV strains are divided into three subgroups, IA, IB and II, based on the sequence of the 5′ untranslated region of the genomic RNA 3. Overall nucleotide sequence similarity among CMV strains is approximately 70%–98%.
Geographical distribution, host range and symptomatology: CMV is distributed worldwide, primarily in temperate to tropical climate zones. CMV infects more than 1200 species of 100 plant families, including monocot and dicot plants. Symptoms caused by CMV infection vary with the host species and/or CMV strain, and include mosaic, stunt, chlorosis, dwarfing, leaf malformation and systemic necrosis. CMV disease is spread primarily by aphid transmission in a nonpersistent manner.
Physical properties: In tobacco sap, the thermal inactivation point of the viral infectivity is approximately 70 °C (10 min), the dilution end‐point is approximately 10−4 and viral infectivity is lost after a few days of exposure to 20 °C. Viral infectivity can be retained in freeze‐dried tissues and in the form of virions purified using 5 mm sodium borate, 0.5 mm ethylenediaminetetraacetic acid and 50% glycerol (pH 9.0) at −20 °C. CMV particles are isometric, approximately 28–30 nm in diameter and are composed of 180 capsid subunits arranged in pentamer–hexamer clusters with T= 3 symmetry. The sedimentation coefficient (s 20,w) is c. 98 S and the particle weight is (5.8–6.7) × 106 Da. The virions contain 18% RNA. The RNA–protein interactions that stabilize the CMV virions are readily disrupted by sodium dodecylsulphate or neutral chloride salts.
Genomic properties: The genomic RNAs are single‐stranded messenger sense RNAs with 5′ cap and 3′ tRNA‐like structures containing at least five open reading frames. The viral RNA consists of three genomic RNAs, RNA 1 (c. 3.3 kb), RNA 2 (c. 3.0 kb) and RNA 3 (c. 2.2 kb), and two subgenomic RNAs, RNA 4 (c. 1.0 kb) and RNA 4A (c. 0.7 kb). The 3′ untranslated regions are conserved across all viral RNAs. CMV is often accompanied by satellite, noncoding, small, linear RNA that is nonhomologous to the helper CMV.
Useful websites: http://www.dpvweb.net/dpv/showdpv.php?dpvno=400 (general information); http://www.rcsb.org/pdb/explore/explore.do?pdbId=1f15 (structure).
INTRODUCTION
Cucumber mosaic virus (CMV), which belongs to the genus Cucumovirus (family Bromoviridae), is an icosahedral plant virus approximately 28–30 nm in diameter (Fig. 1A). The discovery of CMV diseases dates back to approximately 100 years ago. In 1916, Doolittle and Jagger simultaneously reported the first examples of CMV diseases on cucumber and other Cucurbitaceae plants (Doolittle, 1916; Jagger, 1916). Since then, CMV diseases have been reported for over 1200 species in over 100 families of monocots and dicots, including crops, vegetables, ornamentals and woody plants worldwide, especially in temperate to tropical zones. In the environment, CMV is transmitted by at least 75 species of aphid in a nonpersistent manner (Palukaitis et al., 1992). CMV is also transmitted by the parasitic plant dodder (Cuscuta spp.) and, in some cases, through seeds (Palukaitis and García‐Arenal, 2003a). Under experimental conditions, CMV infection frequently has been established by mechanical inoculation using sap, purified virions or viral RNA. CMV causes primarily mosaic (Fig. 1B) and stunt diseases in the families Cucurbitaceae, Solanaceae, Leguminosae, Brassicaceae and Gramineae, and in many other plant species. The disease symptoms are variable and include chlorosis, systemic necrosis, dwarfing and leaf malformation depending on the host species–CMV strain specificities. CMV strains actively propagate in Nicotiana glutinosa and N. tabacum (tobacco), which are used as propagative plants. The mechanical inoculation of CMV causes necrotic local lesions (NLLs) in cowpea (Vigna unguiculata), Chenopodium amaranticolor and C. quinoa, which are useful assay plants.
Figure 1.
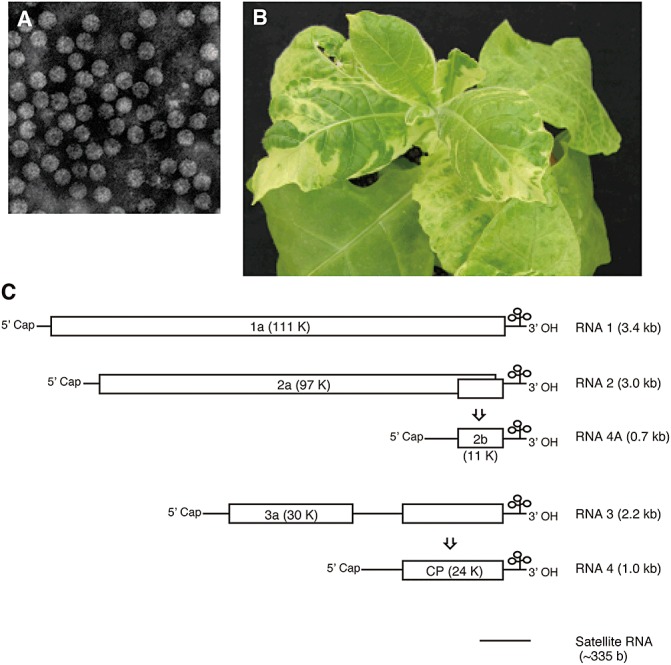
(A) Purified virions of Cucumber mosaic virus (CMV) observed by transmission electron microscope. The sample was negatively stained with uranyl acetate. (B) Mosaic symptoms of CMV‐infected tobacco plant. The uninoculated upper leaves of the tobacco plant are alternately mosaic, then symptomless and, finally, again mosaic. This phenomenon is known as cyclic mosaic symptom expression. (C) Genomic structure of CMV and satellite RNA. The five open reading frames are denoted by boxes.
CMV isolates in the field often contain satellite (sat) RNAs, which are noncoding, small, linear RNAs (Roossinck et al., 1992). The replication and spread of sat RNA in the plant is completely dependent on CMV, and therefore CMV is called a helper virus, although the sequence of this sat RNA is not homologous to that of CMV RNA. sat RNA often facilitates the modification of the symptoms caused by CMV, either by attenuation or by enhancement.
Diverse strains of CMV exist in which the biological properties, such as serology, host range, virulence, resistance induction and aphid transmissibility, are different. CMV strains have been divided into three subgroups, IA, IB and II, based on the sequence of the 5′ untranslated region (UTR) of the genomic RNA 3 (Roossinck et al., 1999). The overall nucleotide sequence similarities among different subgroups are 92%–94% (IA/IB), 74%–78% (IA/II) and 73%–78% (IB/II) (Roossinck, 2001). At present, a number of viral genome sequences for CMV strains have been determined and are present in the National Center for Biotechnology Information (NCBI) database. Importantly, the high genetic diversity of CMV strains has been put forward as an explanation for the differences in biological properties among CMV strains. This diversity provides an opportunity to understand the roles of viral genes in the virulence of CMV. In general, subgroup I strains are more virulent than subgroup II strains; most subgroup I strains develop severe symptoms, including mosaic and stunt, whereas most subgroup II strains show very mild symptoms or asymptomatic infection. The severity of the disease symptoms is also different among subgroup I strains. This review updates the CMV pathogen profile by Roossinck (2001).
The CMV genome consists of three single‐stranded messenger sense RNAs, termed 1, 2 and 3, and also contains subgenomic RNAs, known as RNA 4 and RNA 4A (Fig. 1C). At least five open reading frames (ORFs) are encoded by the CMV genome. Proteins 1a (c. 111 kDa), 2a (c. 97 kDa) and 3a (c. 30 kDa) are translated from the genomic RNAs 1, 2 and 3, respectively, and proteins 2b (c. 15 kDa) and 3b (c. 25 kDa) are translated from subgenomic RNA 4A and RNA 4, respectively. The properties and functional domains of each protein have been described in detail in a review by Palukaitis and García‐Arenal (2003b). The 1a and 2a proteins act as the viral replicase; 1a contains methyltransferase and helicase domains and a capping activity that are crucial for translation, whereas 2a possesses an RNA‐dependent RNA polymerase domain and is solely capable of synthesizing positive‐strand RNAs. Both 1a and 2a are required for negative‐strand synthesis (Seo et al., 2009). The 2b protein acts as a viral suppressor of RNA silencing (VSR) (Béclin et al., 1998; Brigneti et al., 1998) by preventing the propagation of long‐distance signals (Guo and Ding, 2002). The 2b protein inhibits the activities of small interfering RNA (siRNA) and Argonaute (AGO) by direct binding (Goto et al., 2007; Zhang et al., 2006). In addition, the 2b protein facilitates the inhibition of both the salicylic acid (Ji and Ding, 2001) and jasmonic acid (Lewsey et al., 2010) defence pathways, possibly by interfering with the regulation of defence gene expression via RNA silencing. Protein 3a is essential for viral intercellular movement (Canto et al., 1997). It binds, inhibits and severs F‐actin, which have been demonstrated to be essential to increase the plasmodesmata size exclusion limit (Su et al., 2010). Protein 3b is the capsid protein, which is the sole protein associated with the virions and is required for intercellular and long‐distance movement (Canto et al., 1997) and aphid transmission (Perry et al., 1994).
VIRULENCE DETERMINANTS
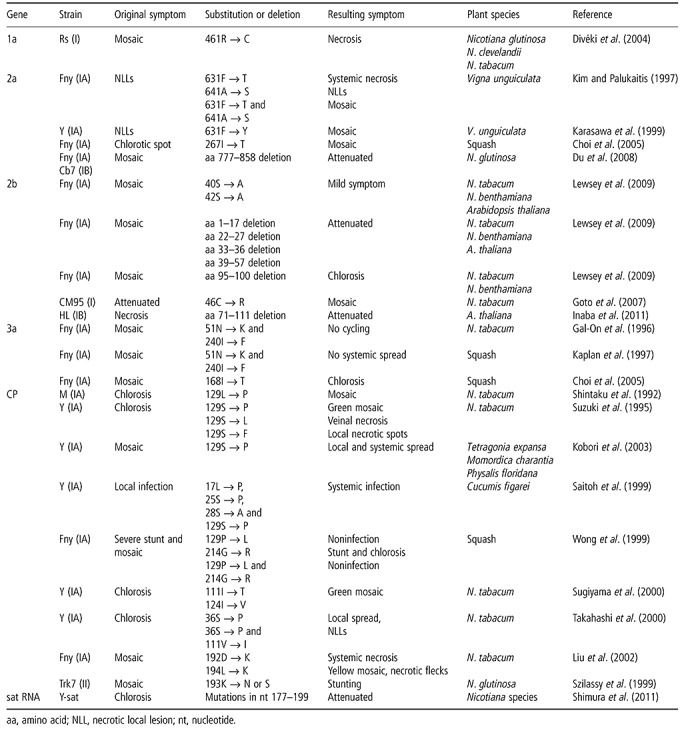
The development of disease is determined by the interactions between the virus and the host plant. On the viral side, qualitative pathogenicity factors and quantitative virulence factors are involved in the development of disease. Because plant viruses encode those genes that are the minimum requirement for infecting plants (Hull, 2002), the majority of plant viral genes act as pathogenicity factors. Indeed, the role of CMV genes in infectivity, which, in turn, facilitates pathogenicity, has been well studied (see above). However, CMV strains display a range of virulences, implying that all CMV genes might also act as virulence factors. Many of the specific amino acids and nucleic acids of CMV genes involved in virulence have been discovered (Table 1), and the roles of CMV genes in virulence have been examined in detail over the last decade. In the following sections, the latest findings on the roles of each CMV gene and sat RNA in CMV virulence are described.
Table 1.
Amino acids and nucleic acids in Cucumber mosaic virus (CMV) genes involved in virulence.
1a protein
The involvement of RNA 1 in virulence has been reported in zucchini squash (Roossinck and Palukaitis, 1990). The differences in the viral kinetics and symptom severities of Fny and Sny strains in zucchini squash are associated with RNA 1 (Gal‐On et al., 1994).
The 1a protein was first reported to be an elicitor of cell death in Nicotiana species. The Ns strain induced NLLs and/or systemic necrotic spots on several Nicotiana species, including N. clevelandii, N. glutinosa and N. tabacum cv. Xanthi. Divéki et al. (2004) showed that an amino acid at position 461 in the 1a protein is the determinant for cell death, i.e. 461C induced cell death in Nicotiana species, but 461R did not. Salánki et al. (2007) further studied a possible correlation between the symptomatic effects of amino acid changes and the secondary structure of the mutated 1a protein using several 1a mutants. There are two amphiphilic α‐helix domains between residues 443 and 472, and amino acid 461 is localized to the boundary between the hydrophilic and hydrophobic faces of the helix. Amino acid substitutions at 461 (461C → E, K, R or P) that changed the integrity and amphiphilicity of the helix affected the viral replication and systemic viral infection. However, the symptomatic effect was unaltered by those amino acid substitutions (461C → A or S) that did not change the properties of the helix (Salánki et al., 2007). Thus, the changes in the integrity and/or surface electric potential of the 1a protein induced by the amino acid mutations at 461 may have led to alterations in the interactions with host factors or the membrane association of the 1a protein, leading to the symptomatic effect. One exception to this speculation is substitution with N: this substitution induced replication deficiency, although the protein retained its original structure and amphiphilicity (Salánki et al., 2007).
The mechanism by which the 1a protein affects CMV virulence is not understood. Recently, a novel methyltransferase from N. tabacum, termed Tcoi1, was determined to be a host protein that methylates the 1a protein by binding directly with the 1a methyltransferase domain (Kim et al., 2008). Interestingly, the methylation of the 1a protein by Toci1 is associated with the systemic spread of CMV, because the overexpression of Tcoi1 enhances systemic infection without affecting replication or intercellular movement.
2a protein
Many CMV strains induce NLLs, in which the virus is restricted by the hypersensitive cell death response. This is not universal, however, as Leguminosae strains can systemically infect cowpea without NLL induction. Kim and Palukaitis (1997) determined that the two amino acids 631F and 641A of the 2a protein are responsible for the elicitation of NLLs, because the Leguminosae strain encodes amino acids 631Y and 641S. The authors showed that the Fny 2a protein with the substitution 641A → S retains the NLL‐eliciting ability of the parental Fny. Similarly, only the 631Y → F substitution in the 2a protein of the Leguminosae strain was sufficient to elicit NLLs in the cowpea cv. ‘Kurodane‐Sanjaku’, whereas NLL elicitation was lost in a 631F → Y mutant of the 2a protein of the Y strain (Karasawa et al., 1999). These two reports indicate that the amino acid 631F in the 2a protein is necessary and sufficient for the elicitation of NLLs. Interestingly, the substitution 631F → Y in the Fny 2a protein induced systemic necrosis in cowpea, indicating that 641A alone can trigger cell death, but does not elicit the viral restriction response (Kim and Palukaitis, 1997). In contrast, only the 631F → Y substitution in the 2a protein of the Y strain in which the 641A residue remained was sufficient to cause the systemic mosaic phenotype in cowpea cv. ‘Kurodane‐Sanjaku’ (Karasawa et al., 1999). Therefore, when NLLs are not elicited, i.e. amino acid 631 in 2a is not F, the symptoms on cowpea leaves may depend on the cultivar–CMV strain specificity. For example, cowpea cv. ‘PI18937’ developed systemic necrosis when infected by the Y strain, but not by pseudo‐recombinant CMV containing RNA 3 from the Leguminosae strain. This indicates that RNA 3 of the Y strain determines systemic necrosis in cv. ‘PI189375’ but not in cv. ‘Kurodane‐Sanjaku’ (Karasawa et al., 1999). The amino acid 641A in the Fny 2a protein may likewise determine systemic necrosis in cowpea (Kim and Palukaitis, 1997).
The involvement of the 2a protein in host‐specific or strain‐specific CMV virulence has been reported. Choi et al. (2005) reported that the 2a protein determined the systemic symptoms on squash. The Fny 2a protein containing 276I produced a systemic mosaic phenotype on squash, whereas the 2a protein of the Pf strain containing 276T produced chlorosis. The differences in symptoms correlated with differences in the rate of intercellular movement in squash, but not in tobacco. Thus, amino acid 276 in the 2a protein mediated the symptom phenotypes by facilitating host‐specific viral movement. Du et al. (2008) found that the deletion of the C‐terminal region of the 2a protein of Fny CMV (subgroup IA) and Cb7 (subgroup IB) strains caused a reduction in the accumulation of viral RNA and the attenuation of symptoms in inoculated N. glutinosa. Because this region of the 2a protein of the Q strain (subgroup II) is not required for virulence and virus accumulation (Ding et al., 1995), it is likely that the C‐terminal region of the 2a protein is involved in enhancing virulence and viral RNA accumulation specifically in subgroup I strains.
2b protein
The 2b protein, a VSR, is an important virulence determinant of CMV. Ding et al. (1995) first showed that the prevention of translation of the 2b protein of the mild Q strain (Q‐Δ2b) caused attenuation in N. glutinosa and a defect in systemic infection in cucumber. The amino acid identity of the 2b protein between the severe subgroup I and asymptomatic subgroup II strains is 53%–54% (Ding et al., 1994), and the 2b protein of subgroup I is 10 amino acids longer than the 2b protein from subgroup II (Ding et al., 1994; Ye et al., 2009). The difference in virulence between subgroups I and II is considered to be a result of differences in the 2b gene (Shi et al., 2002). Since then, much work has been performed to attempt to understand the role of the 2b protein in virulence.
Diaz‐Pendon et al. (2007) showed that the Q‐Δ2b mutant was capable of causing disease in Arabidopsis mutants deficient in the RNA‐silencing component genes DCL2–4. The virulence of the Δ2b mutant of the severe Fny strain was likewise restored in rdr1/6, ago1 and ago2 Arabidopsis mutants (Wang et al., 2011). Because Δ2b accumulated in the systemic leaves of these Arabidopsis mutants, but not in wild‐type Arabidopsis, Diaz‐Pendon et al. (2007) determined that Q‐2b is required for successful systemic infection by the CMV Q strain, but is dispensable for the elicitation of disease symptoms. Our observation of pepo‐Δ2b infection kinetics in tobacco plants revealed the limited distribution of pepo‐Δ2b in developing tissues, including shoot meristem and leaf primordia, suggesting that reduced infection by pepo‐Δ2b results in attenuation (Sunpapao et al., 2009). These findings indicate that 2b plays an indirect role in virulence by facilitating efficient viral systemic infection, especially in growing tissues. Sole expression of 2b protein from a mild strain, which by itself does not show any symptoms, complemented the infection of growing tissues by the unrelated Tobacco mosaic virus (TMV) that did not invade the growing tissues, and this complementation induced more virulent TMV via a synergistic effect (Siddiqui et al., 2011). Interestingly, Diaz‐Pendon et al. (2007) showed that the development of symptoms as a result of infection with the wild‐type Q strain in the dcl2/3/4 Arabidopsis mutant was delayed compared with infection with Δ2b, suggesting that the 2b protein delays the development of severe disease symptoms in the early stages of infection.
The 2b protein is capable of inducing symptoms directly by the perturbation of microRNA (miRNA) pathways and by the inhibition of host protein activity via direct binding (Inaba et al., 2011; 2007, 2009; Zhang et al., 2006). Lewsey et al. (2007) compared the effect on symptom expression of the 2b proteins of the severe Fny and mild LS strains. The authors demonstrated that the constitutive expression of Fny 2b caused a symptom‐like phenotype on Arabidopsis, whereas LS 2b did not. Only Fny 2b could perturb the miRNA‐guided RNA degradation pathway, whereas both Fny and LS 2b proteins equally suppressed siRNA‐mediated RNA degradation. These findings indicate that the 2b protein of severe CMV strains elicits symptoms by direct inhibition of the miRNA pathway, a function that is independent of siRNA‐mediated RNA silencing suppression. Strikingly, Zhang et al. (2006) demonstrated that the Fny 2b protein inhibited AGO1 function via direct binding, which caused the perturbation of the miRNA pathway and resulted in the abnormal development of transgenic Arabidopsis constitutively expressing Fny 2b. More recently, a direct host–virus interaction has been demonstrated between the Arabidopsis catalase 3 (CAT3) and the 2b protein of an HL strain that induced necrosis on inoculated Arabidopsis (Inaba et al., 2011). The C‐terminal region of the 2b protein was essential for binding to CAT3 and for necrosis induction. Because CAT is the antioxidative enzyme that catabolizes H2O2, direct binding between the HL 2b protein and CAT3 depresses CAT activity, leading to a large accumulation of H2O2 that results in cell death.
The functional domains of the 2b protein that facilitate virulence have been investigated by Lewsey et al. (2009). The authors demonstrated that the 2b VSR function of the severe Fny strain alone is insufficient for the induction of symptoms, because the mutation of two putative phosphorylation sites at residues 40 and 42 in the 2b protein resulted in attenuated symptoms without affecting the nuclear localization and siRNA‐binding ability of the 2b protein. The deletion of the putative nuclear localization signal (NLS) and the putative phosphorylation motif of the 2b protein resulted in the loss of the integrity/stability of the 2b protein and of the virulence (Gonsáles et al., 2010). Lewsey et al. (2009) suggested that the accumulation of the 2b protein itself was important for symptom induction. However, Goto et al. (2007) used the 2b proteins of an attenuated isolate (CM95 strain) that encoded cysteine at amino acid 46 (A2b), and of its revertant encoding arginine at amino acid 46 (R2b), and found that this single substitution determined symptom severity. Although both 2b proteins accumulated equally in the plant cells and localized to the nucleus, only R2b showed intense local and systemic VSR activity. R2b showed strong siRNA and long double‐stranded RNA (dsRNA)‐binding activities, whereas A2b did not; therefore, virulence can be correlated with the VSR activity of the 2b protein (Goto et al., 2007).
In conclusion, the 2b protein may play dual roles in virulence. One is an indirect role for the efficient spread of CMV virulence factors, including 2b itself, to all areas of the plant, and this function can be complemented by deficiencies in the plant RNA silencing component genes. The other is a direct role in the elicitation of symptoms in the tissues via the perturbation of miRNA pathways or of plant antioxidant metabolism.
3a protein
The 3a protein has been reported to be a symptom determinant for cycling symptom expression in inoculated tobacco plants (Gal‐On et al., 1996). Following Fny strain infection, the uninoculated upper leaves of tobacco plants developed alternately mosaic, then symptomless, and finally again mosaic, phenotypes, representing differences in viral distribution among the upper leaves. The amino acid substitutions 51N → K and 240I → F in the 3a protein of the Fny strain resulted in chronic infection without the cycling of symptoms (Gal‐On et al., 1996). These substitutions also caused strong accumulation of 3a, suggesting that cycling symptom expression was affected by 3a‐mediated viral movement. However, in squash, both 51N → K and 240I → F substitutions in Fny 3a resulted in reduced systemic movement (Kaplan et al., 1997). Choi et al. (2005) likewise reported that amino acid 168 in the 3a protein is a determinate of systemic symptoms in squash alone. Thus, the effect of 3a amino acid mutations on virulence may be host specific.
Capsid protein
The capsid protein (CP) is an important symptom determinant for CMV. For example, CP of the Y strain has been shown to elicit NLLs in the Arabidopsis ecotype C24 (Takahashi et al., 2001). In particular, amino acid 129 in CP is a well‐studied symptom determinant. Shintaku et al. (1992) first reported that amino acid 129 in CP determines the symptom phenotypes of tobacco plants: 129P induced a pale green mosaic phenotype, whereas 129S induced chlorosis. A series of amino acid substitutions at residue 129 has demonstrated that amino acid 129 alters the symptoms observed in tobacco plants, resulting in pale green mosaic, chlorosis, veinal necrosis and NLLs (Suzuki et al., 1995). Recently, we investigated the role of amino acid properties in symptom determination using CP mutants of the pepo strain in which 129P was substituted with 19 other amino acids (Mochizuki and Ohki, 2011). The results revealed relationships between the amino acid properties of residue 129 and the symptoms observed in tobacco plants: polar acidic mutants (E and D) induced a pale green mosaic phenotype, whereas polar basic mutants (K and H) induced a necrotic phenotype; aliphatic hydrophobic amino acids (I, L and V) induced a necrotic phenotype, and some chlorosis‐inducing mutants contained substitutions with polar uncharged groups (C, S and Q). Interestingly, the substitution of amino acid 129 affected the symptom phenotypes not only in tobacco, but also in various other plant species, such as Tetragonia expansa, Momordica charantia and Physalis floridana (Kobori et al., 2003), indicating that the effect of amino acid 129 substitution was not host specific. Importantly, the effect of the properties of amino acid 129 on symptoms was dependent on the CMV strain, e.g. M and P6 CMV strains encoding 129L induced chlorosis on tobacco leaves (Shintaku et al., 1992), but the 129L pepo mutant induced veinal necrosis (Mochizuki and Ohki, 2011). Indeed, substitutions at residue 129 influenced the symptom phenotypes associated with other amino acid substitutions in CP (Saitoh et al., 1999; Wong et al., 1999). Therefore, it is likely that the effect of CP amino acid 129 on virulence would be influenced by other amino acid sequences. It should be noted that amino acid changes at other residues in CP could likewise alter symptom phenotypes (Liu et al., 2002; Sugiyama et al., 2000; Szilassy et al., 1999; Takahashi et al., 2000).
The mechanism of amino acid 129‐mediated symptom modification has been examined in terms of CP tertiary structure. Smith et al. (2000) determined the crystal structure of Fny CP at a resolution of 3.2 Å. Gellért et al. (2006) calculated the three‐dimensional structure and electrostatic potential patterns of the mutated CP using homology‐ and protein structure‐based computational modelling and analysis. Two reports have stated that residue 129 is located at the first position of the βE–αEF loop (129–136), although Smith et al. (2000) have indicated that amino acid 129 is not in the outermost portion of CP, and Gellért et al. (2006) have asserted that amino acid 129 is on the external surface of the assembled viral particle. Smith et al. (2000) have also suggested that amino acid 129 does not interact directly with host factors, whereas Gellért et al. (2006) have shown that the flexibility of the βE–αEF loop is dependent on the properties of amino acid 129. Taken together, the flexibility of the βE–αEF loop, regulated by amino acid 129, may determine the CP‐mediated symptom modification, co‐ordinating with other properties regulated by the other amino acids, e.g. the phosphorylation of amino acid 214 (Wong et al., 1999).
The mechanism by which amino acid 129 modifies symptoms has been explored. One explanation is that the amino acid substitution 129S → F in CP of the Y strain disrupts the virion assembly and results in the aggregation of CP molecules, which might then elicit necrosis in tobacco plants (Suzuki et al., 1995). We observed the cytopathological features of symptomatic tissues from tobacco plants infected with our 19 mutants, and found that the only difference between pale green and white mosaic (i.e. chlorosis) mutants was the degree of abnormality of the thylakoid membranes (Mochizuki and Ohki, 2011). We consider that structural changes in CP as a result of substitutions at residue 129 may affect the intensity of the stress response of the plant cells that regulates thylakoid membrane synthesis, resulting in different severities of chlorosis in tobacco cells.
sat RNA
Three distinct mechanisms of sat RNA‐mediated symptom modification have been reported.
D strain sat RNA (D‐sat)‐induced lethal systemic necrosis in tomato plants, occurring in Europe, has been demonstrated by Xu and Roossinck (2000). D‐sat causes nuclear DNA fragmentation and chromatin condensation in necrotic tissues, implicating programmed cell death in D‐sat‐induced necrosis. In addition, the authors found that the spatial patterns of necrosis induction, vascular cell development and D‐sat localization in tissues were correlated. D‐sat may alter normal vascular cell development and lead to programmed cell death, probably via xylogenesis or senescence.
Y strain sat RNA (Y‐sat), one of several well‐known sat RNAs, causes bright yellowing in some solanaceous species, including N. benthamiana, tobacco and pepper. Recently, striking reports on the mechanism of Y‐sat‐induced chlorosis have been published separately by Shimura et al. (2011) and Smith et al. (2011). Y‐sat includes a 22‐nucleotide homology sequence that is complementary to the tobacco magnesium protoporphyrin chelatase subunit I gene (ChlI, key enzyme for chlorophyll synthesis), and the amount of ChlI mRNA is reduced in the chlorotic region of Y‐sat‐infected tissues. Tobacco plants in which the expression of ChlI mRNA was repressed by an RNAi construct or by CMV vector showed chlorotic phenotypes similar to transfection with Y‐sat. Interestingly, Y‐sat could not induce chlorosis in plants encoding a ChlI gene that was not homologous to the Y‐sat sequence, and nucleic acid mutations in the region homologous to Y‐sat resulted in a decrease in the bright yellowing symptom. Taken together, these results demonstrate that the degradation of Y‐sat by RNA silencing generates an siRNA containing the 22‐nucleotide ChlI homology sequence, and that the siRNA‐mediated specific repression of ChlI mRNA leads to the inhibition of the chlorophyll biosynthesis pathway, resulting in the bright yellowing symptom.
A possible mechanism of sat RNA‐mediated symptom attenuation has been proposed by Hou et al. (2011). SD strain sat RNA (SD‐sat) causes the attenuation of the CMV SD strain. These authors found that, for CMV SD, the amounts of RNA 4A and its encoded 2b protein were reduced in N. benthamiana tissue infected with SD‐sat. Because the 2b protein is important for CMV virulence (see above), a decrease in level may explain the attenuation of the helper virus. The way in which SD‐sat reduces the accumulation of RNA 4A was not clarified. Because the propagation of sat RNA is completely dependent on the action of CMV, it has been speculated that a specific competition occurs between SD‐sat and RNA 4A in the SD strain. A reduction in viral titre by sat RNA has been reported in many cases (Roossinck et al., 1992). Therefore, RNA 4A may not be the only target: other genomic RNAs may undergo similar sat RNA‐mediated attenuation.
CONCLUSION
A large body of work on CMV virulence has shown that all CMV genes are capable of facilitating the virulence of the virus. Virulence is plant species–CMV strain specific in some cases, such as necrosis (some Nicotiana species in combination with the 1a protein of the N strain), NLLs (cowpea–2a protein and Arabidopsis ecotype C24–CP of the Y strain) and chlorosis (some solanaceous species–Y‐sat). With the exception of these particular cases, it is certain that the 2b protein and CP are general virulence determinants in most host species. It has been shown that the sole expression of 2b of severe strains causes symptom‐like phenotypes and that 2b also contributes indirectly to virulence by promoting viral spread. Such a direct role in virulence has not been shown for CP, although amino acid substitutions in CP have been shown to alter symptom severity. Therefore, there is a possibility that the 2b protein and CP, and perhaps other CMV genes, coordinately facilitate symptom development, as suggested by Lewsey et al. (2009).
REFERENCES
- Béclin, C. , Berthomé, R. , Palauqui, J.C. , Tepfer, M. and Vaucheret, H. (1998) Infection of tobacco or Arabidopsis plants by CMV counteracts systemic post‐transcriptional silencing of nonviral (trans)genes. Virology, 252, 313–317. [DOI] [PubMed] [Google Scholar]
- Brigneti, G. , Voinnet, O. , Li, W.X. , Ji, L.H. , Ding, S.W. and Baulcombe, D.C. (1998) Viral pathogenicity determinants are suppressors of transgene silencing in Nicotiana benthamiana . EMBO J. 17, 6739–6746. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Canto, T. , Prior, D.A.M. , Hellwald, K.‐H. , Oparka, K.J. and Palukaitis, P. (1997) Characterization of Cucumber mosaic virus. IV. Movement protein and coat protein are both essential for cell‐to‐cell movement of Cucumber mosaic virus . Virology, 237, 237–248. [DOI] [PubMed] [Google Scholar]
- Choi, S.K. , Palukaitis, P. , Min, B.E. , Lee, M.Y. , Choi, J.K. and Ryu, K.H. (2005) Cucumber mosaic virus 2a polymerase and 3a movement proteins independently affect both virus movement and the timing of symptom development in zucchini squash. J. Gen. Virol. 86, 1213–1222. [DOI] [PubMed] [Google Scholar]
- Diaz‐Pendon, J.A. , Li, F. , Li, W.‐X. and Ding, S.‐W. (2007) Suppression of antiviral silencing by Cucumber mosaic virus 2b protein in Arabidopsis is associated with drastically reduced accumulation of three classes of viral small interfering RNAs. Plant Cell, 19, 2053–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, S.W. , Anderson, B.J. , Haase, H.R. and Symons, R.H. (1994) New overlapping gene encoded by the Cucumber mosaic virus genome. Virology, 198, 593–601. [DOI] [PubMed] [Google Scholar]
- Ding, S.‐W. , Li, W.‐X. and Symons, R.H. (1995) A novel naturally occurring hybrid gene encoded by a plant RNA virus facilitates long distance virus movement. EMBO J. 14, 5762–5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divéki, Z. , Salánki, K. and Balázs, E. (2004) The necrotic pathotype of the Cucumber mosaic virus (CMV) ns strain is solely determined by amino acid 461 of the 1a protein. Mol. Plant–Microbe Interact. 17, 837–845. [DOI] [PubMed] [Google Scholar]
- Doolittle, S.P. (1916) A new infectious mosaic disease of cucumber. Phytopathology, 6, 145–147. [Google Scholar]
- Du, Z. , Chen, F. , Zhao, Z. , Liao, Q. , Palukaitis, P. and Chen, J. (2008) The 2b protein and the C‐terminus of the 2a protein of Cucumber mosaic virus subgroup I strains both play a role in viral RNA accumulation and induction of symptoms. Virology, 380, 363–370. [DOI] [PubMed] [Google Scholar]
- Gal‐On, A. , Kaplan, I. , Roossinck, M.J. and Palukaitis, P. (1994) The kinetics of infection of zucchini squash by Cucumber mosaic virus indicate a function for RNA 1 in virus movement. Virology, 205, 280–289. [DOI] [PubMed] [Google Scholar]
- Gal‐On, A. , Kaplan, I.B. and Palukaitis, P. (1996) Characterization of Cucumber mosaic virus. II. Identification of movement protein sequences that influence its accumulation and systemic infection in tobacco. Virology, 226, 354–361. [DOI] [PubMed] [Google Scholar]
- Gellért, A. , Salánki, K. , Náray‐Szabó, G. and Balázs, E. (2006) Homology modelling and protein structure based functional analysis of five cucumovirus coat proteins. J. Mol. Graph. Model. 24, 319–327. [DOI] [PubMed] [Google Scholar]
- Gonsáles, I. , Martínez, L. , Rakitina, D.V. , Lewsey, M.G. , Atencio, F. , Llave, C. , Kalinina, N.O. , Carr, J.P. , Palukaitis, P. and Canto, T. (2010) Cucumber mosaic virus 2b protein subcellular targets and interactions: their significance and RNA silencing suppressor activity. Mol. Plant–Microbe Interact. 23, 294–303. [DOI] [PubMed] [Google Scholar]
- Goto, K. , Kobori, T. , Kosaka, Y. , Natsuaki, T. and Masuta, C. (2007) Characterization of silencing suppressor 2b Cucumber mosaic virus based on examination of its small RNA‐binding abilities. Plant Cell Physiol. 48, 1050–1060. [DOI] [PubMed] [Google Scholar]
- Guo, H.‐S. and Ding, S.‐W. (2002) A viral protein inhibits the long range signaling activity of the gene‐silencing signal. EMBO J. 21, 398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, W.‐N. , Duan, C.‐G. , Fang, R.‐X. , Zhou, X.‐Y. and Guo, H.‐S. (2011) Satellite RNA reduces expression of the 2b suppressor protein resulting in the attenuation of symptoms caused by Cucumber mosaic virus infection. Mol. Plant Pathol. 12, 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull, R. (2002) Genome organization In: Matthews' Plant Virology, 4th edn. New York: Academic Press. [Google Scholar]
- Inaba, J.I. , Kim, B.M. , Shimura, H. and Masuta, C. (2011) Virus‐induced necrosis is a consequence of direct protein–protein interaction between a viral RNA silencing suppressor and a host catalase. Plant Physiol. 156, 2026–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger, I.C. (1916) Experiments with the cucumber mosaic disease. Phytopathology, 6, 148–151. [Google Scholar]
- Ji, L.H. and Ding, S.‐W. (2001) The suppressor of transgene RNA silencing encoded by Cucumber mosaic virus interferes with salicylic acid‐mediated virus resistance. Mol. Plant–Microbe Interact. 14, 715–724. [DOI] [PubMed] [Google Scholar]
- Kaplan, I.B. , Gal‐On, A. and Palukaitis, P. (1997) Characterization of Cucumber mosaic virus. III. Localization of sequences in the movement protein controlling systemic infection in cucurbits. Virology, 230, 343–349. [DOI] [PubMed] [Google Scholar]
- Karasawa, A. , Okada, I. , Akashi, K. , Chida, Y. , Hase, S. , Nakazawa‐Nasu, Y. , Ito, A. and Ehara, Y. (1999) One amino acid change in Cucumber mosaic virus RNA polymerase determines virulent/avirulent phenotypes on cowpea. Phytopathology, 89, 1186–1192. [DOI] [PubMed] [Google Scholar]
- Kim, C.H. and Palukaitis, P. (1997) The plant defense response to Cucumber mosaic virus in cowpea is elicited by the viral polymerase gene and affects virus accumulation in single cells. EMBO J. 16, 4060–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M.J. , Huh, S.U. , Ham, B.K. and Paek, K.H. (2008) A novel methyltransferase methylates Cucumber mosaic virus 1a protein and promotes systemic spread. J. Virol. 82, 4823–4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori, T. , Miyagawa, M. , Nishioka, K. , Ohki, S.T. and Osaki, T. (2003) Amino acid 129 of Cucumber mosaic virus coat protein determines local symptom expression and systemic movement in Tetragonia expansa, Momordica charantia and Physalis floridana . J. Gen. Plant Pathol. 68, 81–88. [Google Scholar]
- Lewsey, M. , Robertson, F.C. , Canto, T. , Palukaitis, P. and Carr, J.P. (2007) Selective targeting of miRNA‐regulated plant development by a viral counter‐silencing protein. Plant J. 50, 240–252. [DOI] [PubMed] [Google Scholar]
- Lewsey, M. , Surette, M. , Robertson, F.C. , Ziebell, H. , Choi, S.H. , Ryu, K.H. , Canto, T. , Palukaitis, P. , Payne, T. , Walsh, J.A. and Carr, J.P. (2009) The role of the Cucumber mosaic virus 2b protein in viral movement and symptom induction. Mol. Plant–Microbe Interact. 22, 642–654. [DOI] [PubMed] [Google Scholar]
- Lewsey, M.G. , Murphy, A.M. , Maclean, D. , Dalchau, N. , Westwood, J.H. , Macaulay, K. , Bennett, M.H. , Moulin, M. , Hanke, D.E. , Powell, G. , Smith, A.G. and Carr, J.P. (2010) Disruption of two defensive signaling pathways by a viral RNA silencing suppressor. Mol. Plant–Microbe Interact. 23, 835–845. [DOI] [PubMed] [Google Scholar]
- Liu, S. , He, X. , Park, G. , Josefsson, C. and Perry, K.L. (2002) A conserved capsid protein surface domain of Cucumber mosaic virus is essential for efficient aphid vector transmission. J. Virol. 76, 9756–9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki, T. and Ohki, S.T. (2011) Single amino acid substitutions at residue 129 in the coat protein of Cucumber mosaic virus affect symptom expression and thylakoid structure. Arch. Virol. 156, 881–886. [DOI] [PubMed] [Google Scholar]
- Palukaitis, P. and García‐Arenal, F. (2003a) Cucumber mosaic virus. Descr. Plant Viruses No. 400.
- Palukaitis, P. and García‐Arenal, F. (2003b) Cucumoviruses . Adv. Virus Res. 62, 241–323. [DOI] [PubMed] [Google Scholar]
- Palukaitis, P. , Roossinck, M.J. , Dietzgen, R.G. and Francki, R.I. (1992) Cucumber mosaic virus. Adv. Virus Res. 41, 281–348. [DOI] [PubMed] [Google Scholar]
- Perry, K.L. , Zhang, L. , Shintaku, M.H. and Palukaitis, P. (1994) Mapping determinants in Cucumber mosaic virus for transmission by Aphis gossypii . Virology, 205, 591–595. [DOI] [PubMed] [Google Scholar]
- Roossinck, M.J. (2001) Cucumber mosaic virus, a model for RNA virus evolution. Mol. Plant Pathol. 2, 59–63. [DOI] [PubMed] [Google Scholar]
- Roossinck, M.J. and Palukaitis, P. (1990) Rapid induction and severity of symptoms in zucchini squash (Cucurbita pepo) map to RNA 1 of Cucumber mosaic virus . Mol. Plant–Microbe Interact. 3, 188–192. [Google Scholar]
- Roossinck, M.J. , Sleat, D. and Palukaitis, P. (1992) Satellite RNAs of plant viruses: structures and biological effects. Microbiol. Rev. 56, 265–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roossinck, M.J. , Zhang, L. and Hellwald, K.H. (1999) Rearrangements in the 5′ nontranslated region and phylogenetic analyses of Cucumber mosaic virus RNA 3 indicate radial evolution of three subgroups. J. Virol. 73, 6752–6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh, H. , Fujiwara, M. , Ohki, S.T. and Osaki, T. (1999) The coat protein gene is essential for the systemic infection of Cucumber mosaic virus in Cucumis figarei at high temperature. Ann. Phytopathol. Soc. Jpn. 65, 248–253. [Google Scholar]
- Salánki, K. , Gellért, A. , Náray‐Szabó, G. and Balázs, E. (2007) Modeling‐based characterization of the elicitor function of amino acid 461 of Cucumber mosaic virus 1a protein in the hypersensitive response. Virology, 358, 109–118. [DOI] [PubMed] [Google Scholar]
- Seo, J.K. , Kwon, S.J. , Choi, H.S. and Kim, K.H. (2009) Evidence for alternate states of Cucumber mosaic virus replicase assembly in positive‐ and negative‐strand RNA synthesis. Virology, 383, 248–260. [DOI] [PubMed] [Google Scholar]
- Shi, B.J. , Palukaitis, P. and Symons, R.H. (2002) Differential virulence by strains of Cucumber mosaic virus is mediated by the 2b gene. Mol. Plant–Microbe Interact. 15, 947–955. [DOI] [PubMed] [Google Scholar]
- Shimura, H. , Pantaleo, V. , Ishihara, T. , Myojo, N. , Inaba, J. , Sueda, K. , Burgyán, J. and Masuta, C. (2011) A viral satellite RNA induces yellow symptoms on tobacco by targeting a gene involved in chlorophyll biosynthesis using the RNA silencing machinery. PLoS Pathog. 7, e1002021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintaku, M.H. , Zhang, L. and Palukaitis, P. (1992) A single amino acid substitution in the coat protein of Cucumber mosaic virus induces chlorosis in tobacco. Plant Cell, 4, 751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui, S.A. , Valkonen, J.P. , Rajamäki, M.L. and Lehto, K. (2011) The 2b silencing suppressor of a mild strain of Cucumber mosaic virus alone is sufficient for synergistic interaction with Tobacco mosaic virus and induction of severe leaf malformation in 2b‐transgenic tobacco plants. Mol. Plant–Microbe Interact. 24, 685–693. [DOI] [PubMed] [Google Scholar]
- Smith, T.J. , Chase, E. , Schmidt, T. and Perry, K.L. (2000) The structure of Cucumber mosaic virus and comparison to cowpea chlorotic mottle virus. J. Virol. 74, 7578–7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, N.A. , Eamens, A.L. and Wang, M.‐B. (2011) Viral small interfering RNAs target host genes to mediate disease symptoms in plants. PLoS Pathog. 7, e1002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, S. , Liu, Z. , Chen, C. , Zhang, Y. , Wang, X. , Zhu, L. , Miao, L. , Wang, X.C. and Yuan, M. (2010) Cucumber mosaic virus movement protein severs actin filaments to increase the plasmodesmal size exclusion limit in tobacco. Plant Cell, 22, 1373–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama, M. , Sato, H. , Karasawa, A. , Hase, S. , Takahashi, H. and Ehara, Y. (2000) Characterization of symptom determinants in two mutants of Cucumber mosaic virus Y strain, causing distinct mild green mosaic symptoms in tobacco. Physiol. Mol. Plant Pathol. 56, 85–90. [Google Scholar]
- Sunpapao, A. , Nakai, T. , Dong, F. , Mochizuki, T. and Ohki, S.T. (2009) The 2b protein of Cucumber mosaic virus is essential for viral infection of the shoot apical meristem and for efficient invasion of leaf primordia in infected tobacco plants. J. Gen. Virol. 90, 3015–3021. [DOI] [PubMed] [Google Scholar]
- Suzuki, M. , Kuwata, S. , Masuta, C. and Takanami, Y. (1995) Point mutations in the coat protein of Cucumber mosaic virus affect symptom expression and virion accumulation in tobacco. J. Gen. Virol. 76, 1791–1799. [DOI] [PubMed] [Google Scholar]
- Szilassy, D. , Salánki, K. and Balázs, E. (1999) Stunting induced by Cucumber mosaic cucumovirus‐infected Nicotiana glutinosa is determined by a single amino acid residue in the coat protein. Mol. Plant–Microbe Interact. 12, 1105–1113. [DOI] [PubMed] [Google Scholar]
- Takahashi, H. , Sugiyama, M. , Sukamto, Karasawa, A. , Hase, S. and Ehara, Y. (2000) A variant of Cucumber mosaic virus is restricted to local lesions in inoculated tobacco leaves with a hypersensitive response. J. Gen. Plant Pathol. 66, 335–344. [Google Scholar]
- Takahashi, H. , Suzuki, M. , Natsuaki, K. , Shigyo, T. , Hino, K. , Teraoka, T. , Hosokawa, D. and Ehara, Y. (2001) Mapping the virus and host genes involved in the resistance response in Cucumber mosaic virus‐infected Arabidopsis thaliana . Plant Cell Physiol. 42, 340–347. [DOI] [PubMed] [Google Scholar]
- Wang, X.‐B. , Jovel, J. , Udomporn, P. , Wang, Y. , Wu, Q. , Li, W.‐X. , Gasciolli, V. , Vaucheret, H. and Ding, S.‐W. (2011) The 21‐nucleotide, but not 22‐nucleotide, viral secondary small interfering RNAs direct potent antiviral defense by two cooperative argonautes in Arabidopsis thaliana . Plant Cell, 23, 1625–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, S.M. , Thio, S.S.C. , Shintaku, M.H. and Palukaitis, P. (1999) The rate of cell‐to‐cell movement in squash of Cucumber mosaic virus is affected by sequences of the capsid protein. Mol. Plant–Microbe Interact. 12, 628–632. [Google Scholar]
- Xu, P. and Roossinck, M.J. (2000) Cucumber mosaic virus D satellite RNA‐induced programmed cell death in tomato. Plant Cell, 12, 1079–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, J. , Qu, J. , Zhang, J.F. , Geng, Y.F. and Fang, R.X. (2009) A critical domain of the Cucumber mosaic virus 2b protein for RNA silencing suppressor activity. FEBS Lett. 583, 101–106. [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Yuan, Y.‐R. , Pei, Y. , Lin, S.‐S. , Tuschl, T. , Patel, D.J. and Chua, N.‐H. (2006) Cucumber mosaic virus‐encoded 2b suppressor inhibits Arabidopsis Argonaute1 cleavage activity to counter plant defense. Genes Dev. 20, 3255–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]


