Figure 6.
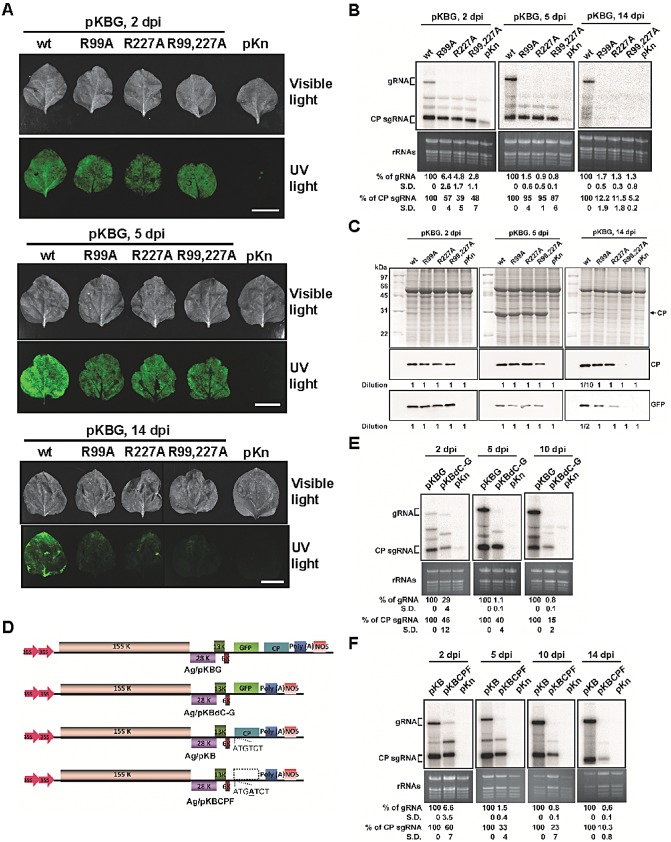
Effects of coat protein (CP) mutations on Bamboo mosaic virus (BaMV) accumulation in Nicotiana benthamiana plants. Leaves were infiltrated with Agrobacterium tumefaciens [optical density at 600 nm (OD600) of 0.1] harbouring pKBG (or pKB) or its derivatives carrying mutant CPs as indicated at the top. (A) Photographs (top panels) and fluorescence images (bottom panels) of infiltrated leaves were taken under visible light or UV illumination at 2, 5 and 14 days post‐inoculation (dpi) (scale bar, 1 cm). The vector backbone pKn was used as a negative control. wt, wild‐type. (B) Total RNAs were analysed by Northern blot hybridization to determine the accumulation of BaMV RNA in infiltrated leaves at 2, 5 and 14 dpi. The relative amounts (%) of viral genomic (g) and CP subgenomic (sg) RNAs, compared with that of the wt, are shown at the bottom. (C) Western blots were performed to detect the accumulation of various CPs and green fluorescent protein (GFP) in agroinfiltrated leaves at 2, 5 and 14 dpi using the respective antibodies as indicated on the right. As a result of the excessive difference in accumulation levels between the wt and mutants at 14 dpi, protein samples of the wt were 10‐ or two‐fold diluted, as indicated at the bottom, for Western blot analyses. (D–F) Effect of deletion of CP or its coding sequence on BaMV RNA accumulation in N. benthamiana. (D) Schematic diagrams of various BaMV‐based clones. The pKn vector was selected for use in Agrobacterium infiltration, as indicated by the ‘Ag/’ prefix. The CP deletion mutant, pKBdC‐G, was constructed by removing the CP open reading frame (ORF) in pKBG. To differentiate the effect of CP and its coding sequence, the CP ORF of pKB was disrupted by the insertion of an extra adenine to create the CP frame‐shift mutant, pKBCPF. (E) Nicotiana benthamiana leaves were infiltrated with A. tumefaciens harbouring pKBG, pKBdC‐G or pKn. Total RNAs were analysed by Northern blot hybridization to determine the accumulation of BaMV RNA in agroinfiltrated leaves at 2, 5 and 10 dpi. The relative amounts of viral RNAs are shown at the bottom. The vector backbone pKn was used as a negative control. Leaves infiltrated with pKBdC‐G became wilted at 14 dpi in all three experiments, and were not included in the Northern blot analyses. (F) Nicotiana benthamiana leaves were infiltrated with A. tumefaciens harbouring pKB, pKBCPF or pKn. Total RNAs were analysed as described above in agroinfiltrated leaves at 2, 5, 10 and 14 dpi. The relative amounts of viral RNAs are shown at the bottom. The percentages shown represent the averages of all quantified samples. Three independent experiments were performed. In each experiment, three (B, E) or four (F) 24–28‐day‐old N. benthamiana plants were used, and the third, fourth and fifth true leaves of each plant were inoculated. On days 2, 5, (10) and 14 post‐inoculation, one inoculated leaf was collected from three plants for analyses. To eliminate the differences in individual plants, the RNAs were extracted from the mixture of the three leaves and analysed by Northern blot hybridization.
