Summary
Background
Multistage, stepwise HIV testing and treatment procedures can result in lost opportunities to provide timely antiretroviral therapy (ART). Incomplete engagement of patients along the care cascade translates into high preventable mortality. We aimed to identify whether a structural intervention to streamline testing and linkage to HIV health care would improve testing completeness, ART initiation, and viral suppression and reduce mortality.
Methods
We did a cluster-randomised, controlled trial in 12 hospitals in Guangxi, China. All hospitals were required to be level 2A county general hospitals and ART delivery sites. We selected the 12 most similar hospitals in terms of structural characteristics, past patient caseloads, and testing procedures. Hospitals were randomly assigned (1:1) to either the One4All intervention or standard of care. Hospitals were randomised in a block design and stratified by the historical rate of testing completeness of the individual hospital during the first 6 months of 2013. We enrolled patients aged 18 years or older who were identified as HIV-reactive during screening in study hospitals, who sought inpatient or outpatient care in a study hospital, and who resided in the study catchment area. The One4All strategy incorporated rapid, point-of-care HIV screening and CD4 counts, and in-parallel viral load testing, to promote fast and complete diagnosis and staging and provide immediate ART to eligible patients. Participants in control hospitals received standard care services. All enrolled patients were assessed for the primary outcome, which was testing completeness within 30 days, defined as completion of three required tests and their post-test counselling. Safety assessments were hospital admissions for the first 90 days and deaths up to 12 months after enrolment. This trial is registered with ClinicalTrials.gov, number NCT02084316.
Findings
Between Feb 24 and Nov 25, 2014, we enrolled 478 patients (232 in One4All, 246 in standard of care). In the One4All group, 177 (76%) of 232 achieved testing completeness within 30 days versus 63 (26%) of 246 in the standard-of-care group (odds ratio 19·94, 95% CI 3·86–103·04, p=0·0004). Although no difference was observed between study groups in the number of hospital admissions at 90 days, by 12 months there were 65 deaths (28%) in the in the One4All group compared with 115 (47%) in the standard-of-care group (Cox proportional hazard ratio 0·44, 0·19–1·01, p=0·0531).
Interpretation
Our study provides strong evidence for the benefits of a patient-centred approach to streamlined HIV testing and treatment that could help China change the trajectory of its HIV epidemic, and help to achieve the goal of an end to AIDS.
Funding
US National Institute on Drug Abuse Clinical Trials Network and China’s National Health and Family Planning Commission.
Introduction
Many patients are lost at each step along the HIV care cascade.1 The current standard-of-care pathway from screening to starting of antiretroviral therapy (ART) in China involves multiple hospital visits, separate blood draws, and protracted waiting periods, resulting in substantial loss to follow-up, delays in diagnosis, incomplete clinical assessment, and late treatment initiation.2,3 In some parts of China, only 43% of those who were HIV-reactive in hospital settings were reported to have received confirmatory testing.3 Furthermore, only 57% of individuals who had confirmed HIV infections received CD4 counts within 6 months.4 Because CD4 counts have been used to determine ART eligibility, nearly 80% of patients with newly identified HIV infections who were eligible for ART were not engaged in timely treatment. These missed opportunities for engagement in HIV care ultimately lead to high mortality.2,5
For people living with HIV to benefit fully from ART, it is crucial that they become aware of their status, have access to counselling, receive treatment, and adhere to their regimens.6 We sought to address this issue by assessing a streamlined pathway for patients from presenting as HIV-reactive during screening to initiating ART. Moreover, we selected Guangxi Zhuang Autonomous Region, south China (hereafter referred to as Guangxi) as the setting for our study because it reported the highest numbers of newly reported HIV and AIDS cases and the highest number of AIDS-related deaths in 2011.5 We did a cluster-randomised controlled trial in 12 hospitals in Guangxi to test the effectiveness of a structural intervention: the One4All strategy. This strategy consisted of a new algorithm incorporating rapid, point-of-care HIV screening and CD4 counts, and in-parallel viral load testing to promote fast and complete diagnosis and staging, followed by immediate counselling and ART initiation for eligible patients.
Methods
Study design and participants
We used a cluster-randomised trial study and selected and randomised 12 similar county hospitals in Guangxi, China, and assigned them to either the intervention (One4All) or control (standard-of-care) group. All hospitals were required to be level 2A county general hospitals and ART delivery sites. We selected the 12 most similar hospitals in in terms of structural characteristics, past patient caseloads, and testing procedures (appendix p 1).3,7
The National Institute on Drug Abuse (NIDA) Protocol Review Board and Data and Safety Monitoring Board, as well as respective institutional review boards of the University of California, Los Angeles, CA, USA, and the National Center for AIDS/STD Control and Prevention at the China CDC reviewed and approved the trial protocol and consent process. The protocol is published on CTNDisseminationLibrary.org, and in a publication by Mao and colleagues.7
We enrolled patients who were HIV-reactive during screening at study hospitals who met eligibility criteria. Eligible patients were aged 18 years or older, had two reactive HIV EIA screening test results, sought inpatient or outpatient care in a study hospital, and resided or intended to reside within the study catchment area. We excluded individuals if they had previously received confirmation of HIV infection in any setting, were prisoners or detainees at the time of screening, or were pregnant.
We enrolled all patients meeting eligibility requirements. After enrolment, we obtained basic demographic information. Participant responses were unlimited, self-reported, and not independently verified. We also obtained self-reported risk behaviour information and self-reported information on how the patient’s HIV was acquired. We informed participants of data collection procedures and provided opportunities to ask questions and opt out of study participation. Patients could refuse to participate or share data. However, we still counted their enrolment toward the total number of study participants. We kept all study data confidential. We did not provide study-related reimbursement for participants or obtain individual informed consent.
Randomisation
The 12 county hospitals were selected by the China study team. The NIDA Data and Statistics Center (The Emmes Corporation) randomly assigned hospitals (1:1) to either the intervention group or the control group with a block design, stratified by the hospitals’ historical rate of testing completeness during the first 6 months of 2013 (either <20% [observed in eight hospitals] or ≥20% [observed in four hospitals]). This was an open-label study.7
Procedures
The One4All intervention included rapid, point-of-care HIV EIA screening and CD4 testing, with in-parallel viral load testing to facilitate completeness of diagnostic assessment and accelerate time to ART initiation for those who were deemed eligible (threshold for treatment at the time of this study was CD4 count of ≤350 cells per μL). The appendix (p 2) shows the differences in the pathways between One4All and standard-of-care groups.
For screening for HIV reactivity, the initial screening test in both the One4All intervention and standard-of-care groups was the Wantai Screening HIV (1+2) Ag&Ab EIA (Beijing Wantai Manufacturer of Infectious Diseases Diagnostics). This screen was followed by two additional screens; in the One4All group this was done with the Determine HIV-1/2 rapid test (Abbott Laboratories) and the InTec HIV rapid test (Xiameng InTec Products, Inc), whereas the standard-of-care group did these screens with different EIAs that varied between sites.
For confirmation of screening results in the One4All group, we used a viral load test. We obtained blood samples for viral load testing immediately after a positive HIV-reactivity test. We sent plasma samples for viral load testing to the provincial Center for Disease Control and Prevention (CDC). Laboratory testing required 10–15 days. We contacted participants and asked them to return to the study hospital to receive their results. For the standard-of-care group we obtained an additional blood sample immediately after screening or on a subsequent study hospital visit and sent it to the local city CDC for western blot confirmatory testing. Because confirmatory testing took about 10–15 days, inpatient participants were usually discharged from the study hospital, and we contacted them later by telephone and asked them to return to the hospital to receive their results.
CD4 cell counts were completed in both study groups. For the One4All group, point-of-care CD4 testing was done at the same time as the viral load blood draw with a point-of-care Pima CD4 Analyzer (Alere Healthcare, Boston, MA, USA) using whole blood samples. Results were available within 30 min. We notified participants of their results as soon as possible, but no later than the next day. For the standard-of-care group, on receipt of western blot results from the city CDC, we asked participants to return to the study hospital for another blood draw for CD4 testing. We sent blood specimens to the city CDC for CD4 testing, which required about 10–15 days. We again asked participants to return to the study hospital to receive their CD4 results.
We obtained blood samples for viral load testing at the study hospital and did viral load testing about 1 year after ART initiation in both study groups.
Outcomes
The primary outcome was testing completeness within 30 days from the date of initial HIV-reactive screening. We defined testing completeness as completion of three required tests and their post-test counselling. These tests comprised the initial HIV screening (one to two tests in the One4All group and two to four in the standard-of-care group), CD4 testing, and confirmatory HIV testing by viral load in the One4All group and by western blot in the standard-of-care group. The secondary outcome was ART initiation within 90 days from the date of initial HIV-reactive screening. We defined ART initiation as the receipt of the first ART prescription. Tertiary outcomes included viral suppression (defined as ≤200 copies per mL) and mortality at 12 month follow-up. In our original protocol, we had planned to compare outcomes between drug users and non-users; however, insufficient numbers of drug users were enrolled, which precluded our ability to do this comparison. Data collection on safety was limited to all patients admitted to hospital during the first 90 days after enrolment and deaths up to 12 months after enrolment.
Statistical analysis
We selected a minimum sample size of 180 participants per group, across 12 hospitals (30 patients per hospital), to achieve 93% power based on a one-sided test (α=0·05) to detect a difference between the group proportions of 0·28; under the alternative hypothesis, we assumed the One4All group proportion to be 0·50 and the control group proportion to be 0·22. This calculation assumed an intraclass correlation within hospitals of 0·082 based on historical testing completeness rates of the 12 study hospitals in a preliminary study done during the first 6 months of 2013.3
The primary outcome was the proportion of participants who achieved testing completeness within 30 days from the initial HIV-reactive screening among all enrolled participants, including those who missed the 30 day follow-up (denominator). The secondary and tertiary outcomes were measured in the same way. For example, the viral suppression outcome was the proportion of participants who achieved viral suppression at 12 months among all enrolled participants, including those who missed the 12 month follow-up and those who refused viral load testing. None of the participants who achieved the endpoints had missing data.
We compared the primary outcome between the two groups with G-side GLIMMIX modelling, because this method adjusts for both the hospital-clustering effect to account for intraclass correlation and baseline participant-level and hospital-level confounding factors. The participant-level factors we included in the model were age, gender, ethnic origin, education, occupation, marital status, HIV transmission route, and treatment setting (ie, whether the patient received treatment in an inpatient or outpatient setting). The hospital-level baseline factor included was historical testing completion rate. To assess the consistency of the primary outcome results, we also used a χ2 test adjusted for clustering to measure the association between treatment group and the primary outcome,8 although this method does not account for baseline confounders. We did secondary analyses of the primary outcome measure as a waiting time analysis, examining time from initial reactive screen to testing completeness. For this waiting time analysis, we censored participants who did not meet the testing completeness criteria at their last follow-up dates. We used Kaplan-Meier curves to display survival differences between the two groups over time.
In comparing differences in baseline characteristics between the two groups, for categorical data, we did a χ2 test adjusted for clustering for binary variables. When baseline categorical variables had more than two responses, we calculated an adjusted p value by referring the observed (unadjusted) χ2 value to the distribution of those obtained by randomly permuting observed treatments between hospitals, with the adjusted p value defined as the proportion of permuted χ2 values at least as large as those observed. To test between-group differences in continuous data, we did a t test adjusted for clustering.8
The analytical methods and the adjustment for clustering and covariates effects we used in the secondary analysis of the primary outcome were also applied to the secondary outcome of ART initiation and the tertiary outcome of viral suppression. We did Kaplan-Meier analyses for the time to testing completeness, initiation of ART, and death. Additionally, for the mortality outcome, we used a random effects Cox (shared frailty) model accounting for the hospital clustering effect to calculate the intervention effect on hazard ratio (HR) of death while also controlling for other covariates. Also for the analysis of mortality, we present two analyses, the first adjusting for and the second not adjusting for CD4 count, because the CD4 test itself was part of the primary outcome measure and not routinely measured at initial HIV screening, predominantly in the standard-of-care group, which often collected blood for testing many days after initial screening. In the adjusted analyses, we attempted to make CD4 count somewhat reflect baseline status by coding it into one of three categories according to the status at the 90 day follow-up (>200 cells per μL, ≤200 cells per μL, or missing if no CD4 test completed). We used all three categories in the mortality analysis. We assessed the proportional hazards assumption for each covariate in the Cox model by examining the cumulative sums of martingale-based residuals,9,10 in a fixed multivariate Cox model.
We did not adjust p values or confidence intervals for multiple testing or multiple comparisons. We analysed all data with SAS version 9.3 and 9.4 software. This trial is registered with ClinicalTrials.gov, number NCT02084316, and is closed to new participants.
Role of the funding source
The Emmes Corporation, under contract from NIDA, analysed data and provided study support. NIDA scientists were actively involved in study design, monitoring study implementation, data collection, analysis, and interpretation, and writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
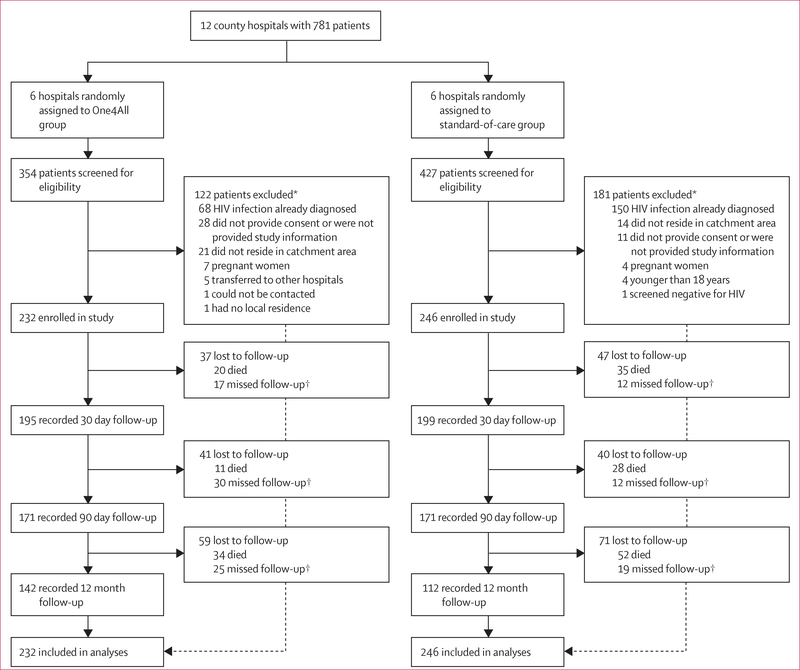
Of 781 patients screened at 12 hospitals in Guangxi, China, between Feb 24 and Nov 25, 2014, we enrolled 478 participants (232 in the One4 All group and 246 in the standard-of-care group; figure 1). Because of the variation in the speed of enrolment across hospitals, several hospitals continued enrolment beyond the target of 30 patients, whereas slower-enrolling hospitals took longer to meet the minimum enrolment target. Follow-up commenced on Feb 24, 2014, and was completed on Jan 16, 2016.
Figure 1: Trial profile.
*Patients may have been ineligible for multiple reasons, thus the number of reasons for ineligibility exceeds the number ineligible. †Patients that missed a follow-up appointment were still invited to provide data at later follow-up timepoints.
Most study participants were male, worked as farmers or labourers, and self-reported heterosexual contact as their route of HIV infection (table 1). Mean age was 54 years (SD 14) in the One4All group and 56 years (14) in the standard-of-care group. More participants in the One4All group had middle school or higher educations (p=0·0498). We did not find any other significant differences in baseline characteristics between the two groups after accounting for hospital clustering effect. Baseline testing completeness (the proportion of patients who, within 30 days of screening HIV-reactive had received western blot confirmatory testing, CD4 cell count testing, and counselling; appendix p 1) was 20 (14%) of 232 patients in the One4All group and 19 (11%) of 246 patients in the standard-of-care group had complete testing at baseline.
Table 1:
Baseline characteristics and outcomes of 30 day testing completeness, 90 day ART initiation, 12 month viral suppression, and 12 month mortality
| Baseline |
Testing completeness, 30
days |
ART initiation, 90
days |
Viral suppression, 12
months |
Mortality, 12 months |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| One4All group | Standard-of-care group | One4All group | Standard-of-care group | One4All group | Standard-of-care group | One4All group | Standard-of-care group | One4All group | Standard-of-care group | |
| Overall | 232 | 246 | 177 (76%) | 63 (26%) | 125 (54%) | 62 (25%) | 99 (43%) | 69 (28%) | 65 (28%) | 115(47%) |
| Age (years) | ||||||||||
| 18–44 | 64 (28%) | 56 (23%) | 57 (89%) | 26 (46%) | 46 (72%) | 24 (43%) | 38 (59%) | 26 (46%) | 9 (14%) | 14 (25%) |
| 45–64 | 112 (48%) | 117 (48%) | 88 (79%) | 27 (23%) | 62 (55%) | 27 (23%) | 47 (42%) | 31 (26%) | 33 (29%) | 56 (48%) |
| ≥65 | 56 (24%) | 73 (30%) | 32 (57%) | 10 (14%) | 17 (30%) | 11 (15%) | 14 (25%) | 12 (16%) | 23 (41%) | 45 (62%) |
| Sex | ||||||||||
| Male | 175 (75%) | 191 (78%) | 132 (75%) | 48 (25%) | 89 (51%) | 46 (24%) | 72 (41%) | 50 (26%) | 56 (32%) | 98 (51%) |
| Female | 57 (25%) | 55 (22%) | 45 (79%) | 15 (27%) | 36 (63%) | 16 (29%) | 27 (47%) | 19 (35%) | 9 (16%) | 17 (31%) |
| Ethnic origin | ||||||||||
| Han | 149 (64%) | 82 (33%) | 122 (82%) | 5 (6%) | 87 (58%) | 12 (15%) | 69 (46%) | 19 (23%) | 48 (32%) | 44 (54%) |
| Zhuang | 77 (33%) | 156(63%) | 49 (64%) | 57 (37%) | 35 (45%) | 49 (31%) | 28 (36%) | 49 (31%) | 17 (22%) | 65(42%) |
| Other | 6 (3%) | 8 (3%) | 6 (100%) | 1 (13%) | 3 (50%) | 1 (13%) | 2 (33%) | 1 (13%) | 0 (0%) | 6 (75%) |
| Marital status | ||||||||||
| Never married | 44 (19%) | 47 (19%) | 35 (80%) | 15 (32%) | 24 (55%) | 10 (21%) | 15 (34%) | 11 (23%) | 12 (27%) | 24 (51%) |
| Married | 139(60%) | 141 (57%) | 108 (78%) | 37 (26%) | 77 (55%) | 39 (28%) | 64 (46%) | 48 (34%) | 34 (24%) | 52 (37%) |
| Divorced or widowed | 49 (21%) | 58 (24%) | 34 (69%) | 11 (19%) | 24 (49%) | 13 (22%) | 20(41%) | 10 (17%) | 19 (39%) | 39 (67%) |
| Education | ||||||||||
| Illiterate | 27 (12%) | 37 (15%) | 16 (59%) | 9 (24%) | 12 (44%) | 6 (16%) | 9 (33%) | 9 (24%) | 11 (41%) | 20 (54%) |
| Primary school | 112 (48%) | 153 (62%) | 81 (72%) | 37 (24%) | 58 (52%) | 33 (22%) | 40 (36%) | 33 (22%) | 37 (33%) | 76 (50%) |
| Middle school or higher | 93 (40%) | 56 (23%) | 80 (86%) | 17 (30%) | 55 (59%) | 23 (41%) | 50 (54%) | 27 (48%) | 17 (18%) | 19 (34%) |
| Principal occupation* | ||||||||||
| Farmer or labourer | 215(93%) | 235 (96%) | 163 (76%) | 61 (26%) | 116 (54%) | 59 (25%) | 92 (43%) | 66 (28%) | 62 (29%) | 108 (46%) |
| Other | 17 (7%) | 11 (4%) | 14 (82%) | 2 (18%) | 9 (53%) | 3 (27%) | 7 (41%) | 3 (27%) | 3 (18%) | 7 (64%) |
| Treatment setting | ||||||||||
| Outpatient | 79 (34%) | 61 (25%) | 74 (94%) | 17 (28%) | 60 (76%) | 21 (34%) | 47 (59%) | 30 (49%) | 11 (14%) | 17 (28%) |
| Inpatient | 153 (66%) | 185 (75%) | 103 (67%) | 46 (25%) | 65(42%) | 41 (22%) | 52 (34%) | 39 (21%) | 54 (35%) | 98 (53%) |
| Route of HIV infection | ||||||||||
| Heterosexual sex | 224(97%) | 238(97%) | 169 (75%) | 62 (26%) | 119 (53%) | 61 (26%) | 93 (42%) | 66 (28%) | 64 (29%) | 111(47%) |
| Other † | 8 (3%) | 8 (3%) | 8 (100%) | 1 (13%) | 6 (75%) | 1 (13%) | 6 (75%) | 3 (38%) | 1 (13%) | 4 (50%) |
| CD4 count (cells per μL)‡ | ||||||||||
| >200 | 65 (28%) | 57 (23%) | .. | .. | 40 (62%) | 23 (40%) | 30 (46%) | 24 (42%) | 9 (14%) | 6 (11%) |
| ≤200 | 141(61%) | 85 (35%) | .. | .. | 85 (60%) | 39 (46%) | 69 (49%) | 39 (46%) | 42 (30%) | 33 (39%) |
| Missing | 26 (11%) | 104 (42%) | .. | .. | 0 (0%) | 0 (0%) | 0 (0%) | 6 (6%) | 14 (54%) | 76 (73%) |
ART=antiretroviral therapy.
Principal occupation response options were the same as those used in China’s National Census Survey; for study purposes, farmers, housekeepers, manual labourers, migrant workers, and long distance truck drivers were grouped together as “Farmer or labourer”; patients who were retirees, educators, health-care providers, unemployed, headsmen, fishermen, students, public service employees, or those who had other or unclear professions, were grouped together as “Other”.
Transmission was by blood donation (n=1), homosexual contact (n=4), injectable drug use (n=6), sexual contact and injectable drug use (n=2), and unknown (n=3).
CD4 count was only categorised for one of the two mortality analyses.
Because the study aimed to assess an intervention intended to improve treatment itself, not treatment as prevention, we did not consider self-reported risk behaviours (appendix p 3) crucial to the assessment of the intervention’s effectiveness.
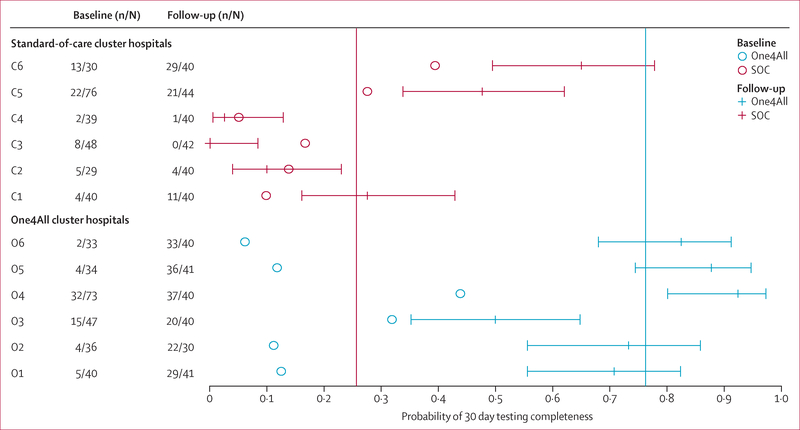
Observed 30 day testing completeness probability outcomes were similar to baseline testing completeness probability in the standard-of-care group, but were much higher in the One4All group (figure 2). These data also suggest a large variability of the testing completeness probability outcomes across the hospitals, particularly within the standard-of-care group. The One4All group had an intraclass correlation of 0–08 compared with 0·28 in the standard-of-care group; an overall intraclass correlation of 0·39.
Figure 2: Forest plot of 30 day testing completeness probability.
Comparison of probabilities of 30 day testing completeness by group and hospital. Circles give historical (baseline) probabilities, while horizontal lines give 30 day outcome probabilities, with 95% CI. Hospitals C1 to C6 represent the SOC control arm hospitals, and O1 to O6 represent One4All intervention arm hospitals. Vertical reference lines show the mean probability of 30 day testing completeness outcomes in the One4All group (blue) and SOC group (red). SOC=standard of care.
In the One4All intervention group, 177 (76%) of 232 participants achieved testing completeness within 30 days compared with 63 (26%) of 246 patients in the standard-of-care group (table 1). After controlling for hospital clustering effect and baseline covariates, odds of testing completeness were significantly higher in the One4All group than in the standard-of-care group (odds ratio [OR] 19·94, 95% CI 3·86–103·04, p=0·0004; table 2). We also observed this large effect in the adjusted χ2 analysis, which controlled for clustering but not the other variables (OR 9·35, 2·54–34·45, p=0·0004). Compared with patients of younger age (18–44 years), patients aged 45–64 years (OR 0·48, 0·24–0·96) and aged 65 years or older (OR 0·24, 0·08–0·74) had decreased odds of testing completeness (overall p=0·044). Compared with patients recruited from outpatient settings, those from inpatient settings had decreased odds of achieving testing completeness (OR 0·31, 0·17–0·55, p=0·0001).
Table 2:
Multivariate models of patients having 30 day testing completeness, 90 day ART initiation, 12 month viral suppression, and 12 month mortality, controlling for hospital clustering
| Testing completeness, 30
days |
ART initiation, 90
days |
Viral suppression, 12
months |
Mortality, 12 months (adjusted
for CD4 count) |
Mortality, 12 months
(unadjusted for CD4 count) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Group | ||||||||||
| One4All | 19·94 (3·86–103·04) | 0·0004 | 3·49 (1·37–8·86) | 0·0087 | 1·59 (0·92–2·73) | 0·094 | 0·62 (0·28–1·36) | 0·23 | 0·44 (0·19–1·01) |
0·053 |
| Standard-of-care | 1 | .. | 1 | .. | 1 | .. | 1 | .. | 1 | .. |
| Age (years) | ||||||||||
| 18–44 | 1 | 0·044 | 1 | 0·0013 | 1 | 0·0066 | 1 | 0·011 | 1 | 0·0002 |
| 45–64 | 0·48 (0·24–0·96) | .. | 0·38 (0·20–0·73) | .. | 0·37 (0·19–0·71) | .. | 2·77 (1·33–3 89) | .. | 2·96 (1·75–5·03) | .. |
| ≥65 | 0·24 (0·08–0·74) | .. | 0·20 (0·08–0·48) | .. | 0·26 (0·11–0·63) | .. | 1·98 (1·08–3·65) | .. | 3 23 (1·79–5·82) | .. |
| Sex | ||||||||||
| Male | 1 | 0·53 | 1 | 0·46 | 1 | 0·88 | 1 | 0·12 | 1 | 0·014 |
| Female | 1·26 (0·61–2·62) | .. | 1·17 (0·77–1·78) | .. | 1·05 (0·55–1·99) | .. | 0·69 (0·44–1·10) | .. | 0·57 (0·36–0·89) | .. |
| Ethnic origin | ||||||||||
| Han | 1 | 0·25 | 1 | 0·12 | 1 | 0·012 | 1 | 0·0003 | 1 | 0·0045 |
| Zhuang | 0·85 (0·47–1·52) | .. | 0·77 (0·41–1·44) | .. | 1·05 (0·64–1·71) | .. | 0·42 (0·24–0·71) | .. | 0·52 (0·30–0·91) | .. |
| Other | 1·70 (0·68–4·24) | .. | 0·25 (0·07–0·93) | .. | 0·30 (0·12–0·71) | .. | 1·97 (0·79–4·90) | .. | 2·12 (0·86–5·23) | .. |
| Marital status | ||||||||||
| Never married | 1 | 0·37 | 1 | 0·018 | 1 | 0·020 | 1 | 0·0005 | 1 | <0·0001 |
| Married | 1·65 (0·81–3·34) | .. | 2·25 (1·22–4·13) | .. | 3·02 (1·38–6·59) | .. | 0·47 (0·30–0·75) | .. | 0·41 (0·26–0·65) | .. |
| Divorced or widowed | 1·24 (0·72–2·13) | .. | 2·13 (1·19–3·79) | .. | 2·43 (1·03–5·76) | .. | 0·80 (0·48–1·35) | .. | 0·80 (0·48–1·32) | .. |
| Education | ||||||||||
| Illiterate | 1 | 0·11 | 1 | 0·44 | 1 | <0·0001 | 1 | 0·032 | 1 | 0·069 |
| Primary school | 1·10 (0·41–2·93) | .. | 1·34 (0·36–5·03) | .. | 0·81 (0·25–2·63) | .. | 1·16 (0·76–1·78) | .. | 0·94 (0·62–1·44) | .. |
| Middle school or higher | 1·91 (0·66–5·55) | .. | 1·93 (0·49–7·50) | .. | 1·92 (0·54–6·76) | .. | 0·66 (0·38–1·16) | .. | 0·58 (0·33–1·02) | .. |
| Principal occupation* | ||||||||||
| Other | 1 | 0·70 | 1 | 0·82 | 1 | 0·22 | 1 | 0·54 | 1 | 0·70 |
| Farmer or labourer | 1·17 (0·54–2·52) | .. | 1·13 (0·40–3·19) | .. | 1·44 (0·80–2·57) | .. | 0·80 (0·39–1·64) | .. | 0·87 (0·43–1·77) | .. |
| Treatment setting | ||||||||||
| Outpatient | 1 | 0·0001 | 1 | 0·0076 | 1 | 0·0007 | 1 | 0·021 | 1 | 0·0003 |
| Inpatient | 0·31 (0·17–0·55) | .. | 0·46 (0·26–0·81) | .. | 0·34 (0·18–0·63) | .. | 1·69 (1·08–2·64) | .. | 2·21 (1·44–3·41) | .. |
| Route of HIV infection | ||||||||||
| Heterosexual sex | 1 | 0·52 | 1 | 0·62 | 1 | 0·097 | 1 | 0·88 | 1 | 0·58 |
| Other† | 0·54 (0·08–3·51) | .. | 1·37 (0·39–4·77) | .. | 2·75 (0·83–9·09) | .. | 0·93 (0·35–2·45) | .. | 0·77 (0·30–1·96) | .. |
| CD4 count (cells per μL)‡ | ||||||||||
| >200 | .. | .. | .. | .. | .. | .. | 1 | <0·0001 | .. | .. |
| ≤200 | .. | .. | .. | .. | .. | .. | 2·76 (1·56–4·48) | .. | .. | .. |
| Missing | .. | .. | .. | .. | .. | .. | 8·26 (4·49–15·71) | .. | .. | .. |
Hospital clustering effect was significant in all multivariate models (p=0’0024 for the model testing viral suppression and p<0’0001 for the others). Baseline hospital testing completeness rate was controlled for in all models. ART=antiretroviral therapy. OR=odds ratio. HR=hazard ratio.
Principal occupation response options were the same as those used in China’s National Census Survey. For the study purposes, farmers, housekeepers, manual labourers, migrant workers, and long distance truck drivers were grouped together as “Farmer or Labor”; patients who were retirees, educators, health-care providers, unemployed, headsmen, fishermen, students, public service employees, or those who had other or unclear professions, were grouped together as “Other”.
Transmission was by blood donation (n=1), homosexual contact (n=4), injectable drug use (n=6), sexual contact and injectable drug use (n=2), and unknown (n=3).
CD4 count was only adjusted for one of the two mortality analyses.
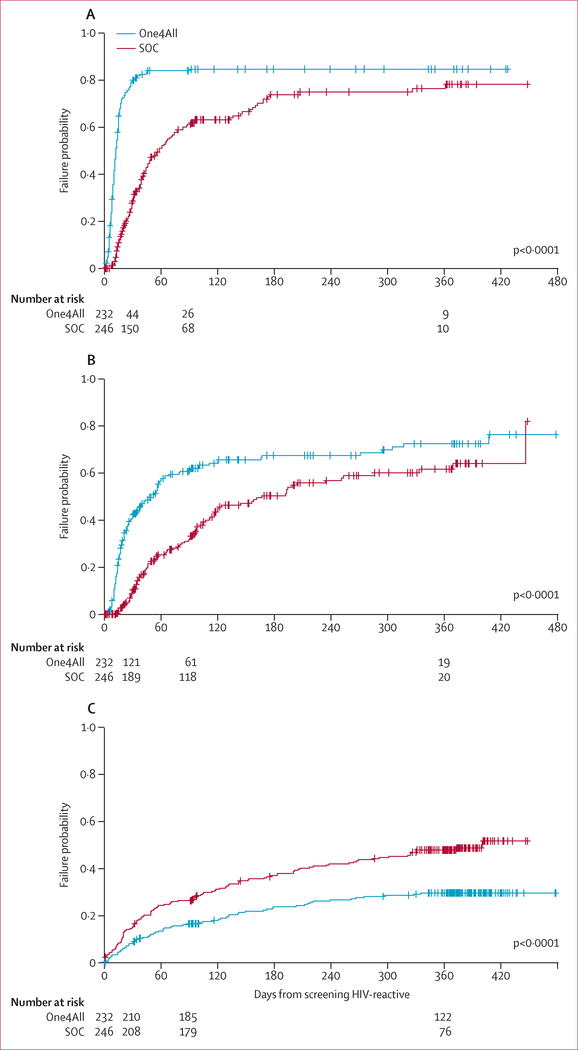
The beneficial effect of the One4All testing algorithm is also supported by time to testing completeness KaplanMeier curves (figure 3), suggesting that participants in the One4All group achieved testing and counselling completion sooner than did participants in the standard-of-care group, with a median of 12 days (IQR 8–24 days) in the One4All group compared with 58 days (IQR 28–207) in the standard-of-care group.
Figure 3: Kaplan-Meier plot for time to testing completeness (A), ART initiation (B), and death (C) from HIV screening.
SOC=standard of care. ART=antiretroviral therapy.
In the One4All group, 125 (54%) of 232 patients initiated ART within 90 days of the date of HIV screening compared with 62 (25%) of 246 in the standard-of-care group (table 1). From the GLIMMX model, One4All group participants had increased odds of 90 day ART initiation compared with standard-of-care group participants (OR 3·49, 95% CI 1·37–8·86, p=0·0087). Three factors, age, marital status, and treatment setting, were predictive of ART initiation within 90 days (table 2). Compared with the youngest age group (18–44 years), older age groups were less likely to initiate ART in 90 days. Being an inpatient at the time of screening was associated with decreased odds of initiating ART within 90 days. Participants who were married or divorced or widowed had decreased odds of initiating ART within 90 days compared with those who had never been married (p=0·0176; table 2). Kaplan-Meier curves (figure 3) show that participants in the One4All group initiated ART sooner than the standard-of-care group. The median time to ART initiation was 52 days (IQR 16–407) in the One4All group compared with 167 days (IQR 57–446) in the standard-of-care group.
Viral suppression (≤200 copies per mL) at 12 months was achieved by 99 (43%) of 232 participants in the One4All group and 69 (28%) of 246 participants in the standard-of-care group (table 1), but the difference was not significant (OR 1·59, 95% CI 0·92–2·73, p=0·094) on the basis of the GLIMMIX model adjusting for clustering effect and baseline characteristics (table 2). Mortality at 12 months was lower in the One4All group than in the standard-of-care group (65 [28%] of 232 vs 115 [47%] of 246; table 1). Specifically, participants in the One4All group had a risk of death lower than that in the standard-of-care group (HR 0·44, 95% CI 0·19–1·01, p=0·053) on the basis of a Cox model controlling for hospital clustering effect and characteristics covariates, but not CD4 count (table 2).
In the model without adjusting for CD4 count, participants’ baseline characteristics significantly associated with a decreased mortality included being of Zhuang origin compared with Han and being married compared with never having been married. Participants’ baseline characteristics significantly associated with an increased death rate included older age compared with younger age (18–44 years), and being an inpatient compared with being an outpatient at the time of initial screening (table 2). We found that the proportional hazard assumptions were satisfied for all covariates adjusted in the Cox model.
We obtained causes of death overall and by study group (appendix p 3). We summarised predictors of time to death since HIV screening in table 2. A low CD4 count within 90 days was a strong predictor of mortality (overall adjusted p<0·0001) in the mortality analysis accounting for CD4 test results. As of the 90 day follow-up, 141 (61%) of 232 participants in the One4All group had CD4 counts of 200 cells per μL or less, and 26 (11%) had not received CD4 tests (table 1). By contrast, 85 (35%) of 246 participants in the standard-of-care group had CD4 counts of 200 cells per μL or less, and 104 (42%) had not received CD4 tests. Those with missing CD4 test results in both groups had the highest mortality with 14 (54%) of 26 participants in the One4All group and 76 (73%) of 104 patients in the standard-of-care group; patients with a missing CD4 count had over eight times higher risk of death than did patients with counts of more than 200 cells per μL (table 2). Kaplan-Meier curves (figure 3C) show that participants in the One4All group had a longer median survival time (>480 days) than did participants in the standard-of-care group (400 days).
A total of 87 participants (18%) of 478 had at least one hospital admission within 90 days—54 (23%) of 232 in the One4All group and 33 (13%) of 246 in the standard-of-care group—a difference that was not significant after accounting for hospital clustering effect (p=0·1078, adjusted χ2 test).
Discussion
Our cluster-randomised controlled trial assessing the One4All diagnostic approach in patients with HIV-reactivity in 12 hospitals in Guangxi, China showed that the extensively streamlined pathway for patients from screening HIV-reactive to initiating ART resulted in 20 times increased testing completeness and 3·5 times increased ART initiation and cut mortality by more than half. Although our results are encouraging, our study highlights the challenging nature of HIV care cascade linkage and retention in China. Even with the elimination of traditional confirmatory HIV testing and addition of point-of-care CD4 testing, 26 participants in the One4All group still did not have a CD4 test by 90 days. More must be done, particularly if China is to meet the UNAIDS 90–90-90 targets.11 China’s removal of CD4 count-based ART eligibility last year12 and recent feasibility testing of point-of-care viral load testing in other low-income and middle-income countries,13 suggest that a model such as One4All that allows for ART initiation on the same day as diagnosis might soon be possible in China.
Despite the intuitive nature of the idea that a patientcentred, streamlined process would promote retention in the HIV care cascade, a systematic review14 of the literature up to February, 2013, found that assessments of this type of intervention were quite rare in low-income and middle-income countries.14 Two studies, one in Lesotho communities15 and one in a hospital setting in Uganda,16 assessing the effects of changes in HIV testing and counselling procedures, found increased testing uptake but no significant effect on linkage to care, ART initiation, or mortality. Another study in primary health-care clinics in South Africa17 found that HIV provider-initiated testing and counselling, which was added to standard care at clinics specialising in the treatment of sexually transmitted infections, improved time to viral load testing, but had no effect on the time to CD4 testing, and ART initiation was not assessed.17 A study in primary health-care clinics in Mozambique18 examined the effect of point-of-care CD4 testing on HIV treatment and found improved time to ART initiation. A study in primary health-care clinics in South Africa found point-of-care CD4 testing increased the likelihood of ART initiation.19 In a mobile voluntary HIV counselling and testing setting in South Africa,20 point-of-care CD4 testing improved linkage to HIV services. However, in general, most studies did not follow up participants beyond the pre-ART period after ART initiation,14 and none assessed the effectiveness of intervention combinations. One study published in 2016 from South Africa21 examined the effect of a rapid ART initiation procedure in clinical and hospital settings and found that 97% of patients initiated ART within 1 month, 73% on the same day, and of those who attended their 6 month follow-up visit, 91% had achieved viral suppression. Furthermore, an as yet unpublished trial done in Haiti22 found that a same-day treatment initiation intervention resulted in improved rates of both ART initiation and retention in care.
In China, understanding the HIV epidemic and how it is evolving over time, including barriers to testing, linkage to HIV treatment, and the causes of attrition along the continuum of care has been a priority for some time. To that end, most studies on China’s HIV epidemic to date have been observational. The characterisation of the HIV epidemic in Guangxi that preceded this study is one example.3 The HIV epidemic in Guangxi is particularly dire with newly identified people with HIV having gone undiagnosed already for an extended period of time as evidenced by the high proportion of people immediately meeting ART eligibility criteria on HIV diagnosis,3,5,23 and the high mortality observed in this report. As another example, a study examining baseline characteristics of ART recipients over the course of nearly 10 years showed that HIV was being increasingly spread by sexual contact in China,24 much like was reported for the current study population. In a further example, a recent nationwide, retrospective study in China,4 which included the records of more than 388 000 people with HIV, found that 57% had first-time CD4 testing within less than 6 months of HIV diagnosis and that 59% already had counts of fewer than 350 cells per μL on their first ever CD4 test. Additionally, a retrospective study in Yunnan province found that in people with newly acquired HIV infections, earlier ART resulted in accelerated recovery of CD4 T-lymphocyte populations. This early, favourable immunological response could help improve clinical outcomes via delayed disease progression.25
Studies assessing the effectiveness of interventions on testing uptake, linkage to care, and retention in treatment have only recently been done in China. The first such large interventional trial in China in 2010–14 made use of a before and after study design. The study examined the benefit of a simplified test-and-treat intervention in which HIV confirmatory testing and CD4 testing were done in parallel and patients were given ART immediately after receipt of test results, irrespective of their CD4 count.2 Not surprisingly, this study found reduced time to HIV confirmation, receipt of CD4 testing, and ART initiation, as well as decreased overall mortality rates. Initiation of ART for all people with HIV, regardless of CD4 count, has been examined in two recently completed clinical trials.26,27 Both of these trials found significant benefit in a treatment-for-all scheme in the form of reduced clinical disease progression.26,27
Our results are not only supported by, but also build on, the abovementioned findings from sub-Saharan Africa,15–21 Haiti,22 and China.2,4,25 Taken together with clinical trial results showing benefit to treating all people with HIV regardless of CD4 count or clinical stage,26,27 our results strongly suggest that a change in approach to provision of treatment for HIV is warranted. Moreover, a study published during the preparation of our manuscript28 found that the One4All strategy was highly cost-effective, and remained so when tested under various scenarios related to changes in the HIV epidemic over time.
The main strength of our study lies in the rigour with which we tested the effectiveness of the One4All intervention, making it the first trial, to our knowledge, to examine a bundle of process improvements to the testing and linkage to care pathway in China. Nevertheless, our trial had limitations. First, this trial design includes few hospitals with high between-cluster variation. Although there was an overall 50% increase in testing completeness in the One4All group compared with the standard-of-care group, the CI for the treatment effect was very wide. This imprecision is at least partly due to the high intraclass correlation found in the standard-of-care group. Second, the clustered study design has disadvantages and caution should be used when generalising to the individual level.29 Third, although the One4All treatment did seem to affect viral suppression positively at 12 months, the study was not sufficiently powered for this outcome. Fourth, since multiple interventions were implemented as a bundle, they cannot be assessed individually. Fifth, the lower level of education, higher inclusion of minorities, and greater proportion of inpatients in the standard-of-care group might have created bias, although these factors were controlled for in the analyses. Finally, the One4All intervention was designed to address challenges in testing and linkage to HIV care in China and, therefore, these specific results might not be directly generalisable to other countries. Yet, an intervention like this can have results similar to what we have found even in a different context, such as in pregnant women in rural Nigeria,30 where point-of-care CD4 testing and integrated services improved ART initiation and retention in care.
Although we report both mortality results with and without adjusting for the CD4 count, there are limitations in both analyses since concentration of CD4 positivity is not truly a baseline measure in this study. For example, CD4 count is an important indicator of the HIV disease stage, and imbalances between treatment groups in baseline CD4 count could confound the treatment group association with mortality. However, because the CD4 test is part of the primary outcome of this trial and is measured after HIV screening, it could be argued that the treatment group assignment itself could affect CD4 test results, arguing against the inclusion of CD4 testing in the statistical model. Therefore, we chose to present both analyses, noting that both show a protective effect of the One4All group compared with the standard-of-care group, with the analysis not accounting for CD4 count of borderline significance.
In conclusion, the results of this prospective, clusterrandomised trial show that implementation of a package of interventions aimed at streamlining the patient pathway from screening HIV-reactive to initiating treatment substantially increased the odds of achieving testing completeness within 30 days, ART initiation within 90 days, and survival at 12 months in hospital settings in China. Taken together with new, strong evidence of the benefits of treating all people with HIV regardless of CD4 level,2,26,27 a patient-centred approach to streamlined HIV testing and ART initiation regardless of CD4 count is clearly beneficial.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed with one of the terms “HIV test” or “HIV diagnosis” in combination with one or more of the following terms: “CD4 test”, “point-of-care”, “ART”, “care continuum”, “linkage to care”, “viral load”, “intervention”, and “mortality”. We restricted our search to articles published between Jan 1, 2000, and Dec 31, 2016, reporting on studies completed in low-income and middle-income countries that were not limited to individual high-risk populations (eg, pregnant women or men who have sex with men). Within these search results, we looked for studies specifically examining effectiveness of structural interventions aimed at changing the patient pathway from testing to treatment that were intended to promote completion of HIV diagnostic testing, linkage to pre-antiretroviral therapy (ART) care, or initiation of ART, without outcome variables of increased testing of CD4 counts, ART initiation, viral load testing, or a reduction in mortality. We found seven studies from sub-Saharan Africa, one from Haiti, and one from China. Two studies, one from Lesotho and one from Uganda, examined changes to HIV testing and counselling procedures and found a higher testing uptake with home-based services or abbreviated protocols, but no improvement in linkage to care, initiation of ART, or a reduction in mortality. A third study examined provider-initiated testing and counselling at sexually transmitted infection clinics in South Africa and found improved time to viral load testing, but no improvement in time to testing of CD4 counts. Three additional studies, one from Mozambique and two from South Africa, provide evidence of the benefit of point-of-care tests for CD4 count in terms of linkage to HIV care, increased odds of ART initiation, and decreased time to ART initiation. More recently, a study of a rapid ART initiation procedure in South Africa found that 97% of patients eligible for treatment began ART within 1 month, 73% on the same day, and of those followed up at 6 months, 91% had achieved viral suppression. Additionally, an as yet unpublished trial of same-day treatment in Haiti found improvements in both ART initiation and post-ART retention in care. The one study done in Asia was completed by the authors of this report, also in Guangxi, China. In this study, a streamlined and standardised timeframe for HIV diagnosis was combined with expanded treatment to cover all patients diagnosed with HIV improved timeliness of testing, time from diagnosis to treatment initiation, proportion of patients initiating treatment, and reduced mortality.
Added value of this study
To our knowledge, this study is the first to assess the effectiveness of a streamlined process for testing and treatment initiation within the rigour of a cluster-randomised design in a middle-income country. We took an innovative approach to addressing barriers to testing and treatment and reasons for attrition along the continuum of care, implementing a tailored package of interventions that targeted multiple points in the process at the same time. The results observed, a 20 times greater chance of achieving testing completeness by 30 days, 3 times greater chance of ART initiation by 90 days, and mortality reduced by 56% at 12 months, far exceeded our expectations and clearly show the benefit of rethinking the path to treatment from a patient-centred perspective.
Implications of all the available evidence
The results we present here, together with previous findings from China and other international settings, provide strong evidence for the benefits of a patient-centred approach to streamlined HIV testing and initiation of ART regardless of CD4 count. Given the size of China, and the growth rate of the country’s HIV epidemic, policy changes such as these could help China to achieve the UNAIDS 90–90-90 target and have a very meaningful effect on the lives of perhaps hundreds of thousands of people. Furthermore, similarly designed, simplified testing and treatment procedures tailored to the unique challenges of different settings along with elimination of CD4 count treatment eligibility requirements could also have profound effects in other low-income and middle-income countries. Although many challenges remain, this could finally put an AIDS-free generation within reach.
Acknowledgments
The study was co-funded by NIDA Clinical Trials Network (U10 DA013045), and the National Health and Family Planning Commission, the People’s Republic of China (131–16-000–105-01; 2012ZX10001–007). The views and opinions expressed in this Article are entirely those of the authors, and do not represent the official policy or endorsement of the National Center for AIDS/STD Control and Prevention, China CDC, or the National Institute on Drug Abuse, US National Institutes of Health. The authors would like to thank all participants, hospital staff, and local and provincial CDC workers involved in this study. The authors would also like to thank Betty Tai and Shicheng Yu for their help in developing the protocol, and Yuejiao Zhou, Xinhua Wu, Shujia Liang, Shuaifeng Liu, Fuxiong Liang, Juan Xu, Xiaoai Qian, Peili Wu, and Ran Xiong for participating in data collection.
Footnotes
Declaration of interests
We declare no competing interests.
Contributor Information
Zunyou Wu, The National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
Zhenzhu Tang, Guangxi Center of Disease Control and Prevention, Nanning, China.
Yurong Mao, The National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
Paul VanVeldhuisen, The Emmes Corporation, Rockville, MD, USA.
Walter Ling, Integrated Substance Abuse Programs, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA.
David Liu, National Institute on Drug Abuse, National Institutes of Health, Bethesda, MD, USA.
Zhiyong Shen, Guangxi Center of Disease Control and Prevention, Nanning, China.
Roger Detels, Department of Epidemiology, UCLA Fielding School of Public Health, Los Angeles, CA, USA.
Guanghua Lan, Guangxi Center of Disease Control and Prevention, Nanning, China.
Lynda Erinoff, National Institute on Drug Abuse, National Institutes of Health, Bethesda, MD, USA.
Robert Lindblad, The Emmes Corporation, Rockville, MD, USA.
Diane Gu, The National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
Houlin Tang, The National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
Lian Hu, The Emmes Corporation, Rockville, MD, USA.
Qiuying Zhu, Guangxi Center of Disease Control and Prevention, Nanning, China.
Li Lu, The Emmes Corporation, Rockville, MD, USA.
Neal Oden, The Emmes Corporation, Rockville, MD, USA.
Albert L Hasson, Integrated Substance Abuse Programs, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA.
Yan Zhao, The National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
Jennifer M McGoogan, The National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
Xianmin Ge, Guangxi Center of Disease Control and Prevention, Nanning, China.
Nanci Zhang, The National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
Keming Rou, The National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
Jinhui Zhu, Guangxi Center of Disease Control and Prevention, Nanning, China.
Hui Wei, The Bureau of HIV/AIDS Control, Guangxi Zhuang Autonomous Regional Health and Family Planning Commission, Nanning, China.
Cynthia X Shi, The National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
Xia Jin, The National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
Jian Li, The National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China.
Julio S G Montaner, BC Center for Excellence in HIV/AIDS, University of British Columbia, Vancouver, BC, Canada.
References
- 1.Nash D, Wu Y, Elul B, Hoos D, El Sadr W. Program-level and contextual-level determinants of low-median CD4+ cell count in cohorts of persons initiating ART in eight sub-Saharan African countries. AIDS 2011; 25: 1523–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z, Zhao Y, Ge X, et al. Simplified HIV testing and treatment in China: analysis of mortality rates before and after a structural intervention. PLoS Med 2015; 12: e1001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu D, Mao Y, Tang Z, et al. Loss to follow-up from HIV screening to ART initiation in rural China. PLoS One 2016; 11: e0164346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang H, Mao Y, Shi CX, et al. Baseline CD4 cell counts of newly diagnosed HIV cases in China: 2006–2012. PLoS One 2014; 9: e96098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Center for AIDS/STD Control and Prevention. Analysis of HIV/AIDS testing and treatment program in Guangxi. Beijing: China CDC, 2012. [Google Scholar]
- 6.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011; 52: 793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao Y, Wu Z, McGoogan JM, et al. Care cascade structural intervention versus standard of care in the diagnosis and treatment of HIV in China: a cluster-randomized controlled trial protocol. BMC Health Serv Res 2017; 17: 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donner A, Klar N. Design and analysis of cluster randomization trials in health research. New York: Oxford University Press, 2000. [Google Scholar]
- 9.Therneau TM, Grambsch PM, Fleming TR. Martingale-based residuals for survival models. Biometrika 1990; 77: 147–60. [Google Scholar]
- 10.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika 1993; 80: 557–72. [Google Scholar]
- 11.Joint United Nations Programme on HIV/AIDS. 90–90-90—an ambitious treatment target to help end the AIDS epidemic. Geneva: UNAIDS, 2014. [Google Scholar]
- 12.Wu Z, Pisani E. Building on the past, facing the future In: Wu Z, ed. HIV/AIDS in China—beyond the numbers. Beijing: People’s Medical Publishing House, 2016: 138–49. [Google Scholar]
- 13.Moyo S, Mohammed T, Wirth KE, et al. Point-of-care Cepheid Xpert HIV-1 viral load test in rural African communities is feasible and reliable. J Clin Microbiol 2016; 54: 3050–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Govindasamy D, Meghij J, Kebede Negussi E, Clare Baggaley R, Ford N, Kranzer K. Interventions to improve or facilitate linkage to or retention in pre-ART (HIV) care and initiation of ART in low- and middle-income settings—a systematic review. J Int AIDS Soc 2014; 17: 19032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labhardt ND, Motlomelo M, Cerutti B, et al. Home-based versus mobile clinic HIV testing and counseling in rural Lesotho: a cluster-randomized trial. PLoS Med 2014; 11: e1001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wanyenze RK, Hahn JA, Liechty CA, et al. Linkage to HIV care and survival following inpatient HIV counseling and testing. AIDS Behav 2011; 15: 751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leon N, Mathews C, Lewin S, Osler M, Boulle A, Lombard C. A comparison of linkage to HIV care after provider-initiated HIV testing and counselling (PITC) versus voluntary HIV counselling and testing (VCT) for patients with sexually transmitted infections in Cape Town, South Africa. BMC Health Serv Res 2014; 14: 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jani IV, Sitoe NE, Alfai ER, et al. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: an observational cohort study. Lancet 2011; 378: 1572–79. [DOI] [PubMed] [Google Scholar]
- 19.Faal M, Naidoo N, Glencross DK, Venter WD, Osih R. Providing immediate CD4 count results at HIV testing improves ART initiation. J Acquir Immune Defic Syndr 2011; 58: e54–59. [DOI] [PubMed] [Google Scholar]
- 20.Larson BA, Schnippel K, Ndibongo B, et al. Rapid point-of-care CD4 testing at mobile HIV testing sites to increase linkage to care: an evaluation of a pilot program in South Africa. J Acquir Immune Defic Syndr 2012; 61: e13–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosen S, Maskew M, Fox MP, et al. Initiating antiretroviral therapy for HIV at a patient’s first clinic visit: the RapIT randomized controlled trial. PLoS Med 2016; 13: e1002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koenig S, Dorvil N, Severe P, et al. Same-day HIV testing and antiretroviral therapy initiation results in higher rates of treatment initiation and retention in care. 21st International AIDS Conference; Durban, South Africa; July 18–22, 2016 Abstr WEAE0202. [Google Scholar]
- 23.National Center for AIDS/STD Control and Prevention. 2011 annual report of national AIDS/STD statistics on epidemics and program implementation. Beijing: China CDC, 2012. [Google Scholar]
- 24.Dou Z, Chen RY, Xu J, et al. Changing baseline characteristics among patients in the China National Free Antiretroviral Treatment Program, 2002–09. Int J Epidemiol 2010; 39 (suppl 2): ii56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding Y, Duan S, Wu Z, et al. Timing of antiretroviral therapy initiation after diagnosis of recent human immunodeficiency virus infection and CD4(+) T-cell recovery. Clin Microbiol Infect 2016; 22: 290.e5–8. [DOI] [PubMed] [Google Scholar]
- 26.Danel C, Moh R, Gabillard D, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015; 373: 808–22. [DOI] [PubMed] [Google Scholar]
- 27.Joint United Nations Programme on HIV/AIDS. Implications of the START study data: questions and answers. Geneva: UNAIDS, 2015. [Google Scholar]
- 28.Zang X, Tang H, Min JE, et al. Cost effectiveness of the ‘One4All’ HIV linkage intervention in Guangxi Zhuang autonomous region, China. PLoS One 2016; 11: e0167308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donner A, Klar N. Pitfalls of and controversies in cluster randomization trials. Am J Public Health 2004; 94: 416–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aliyu MH, Blevins M, Audet CM, et al. Integrated prevention of mother-to-child HIV transmission services, antiretroviral therapy initiation, and maternal and infant retention in care in rural north-central Nigeria: a cluster-randomised controlled trial. Lancet HIV 2016; 3: e202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.