FIG 2.
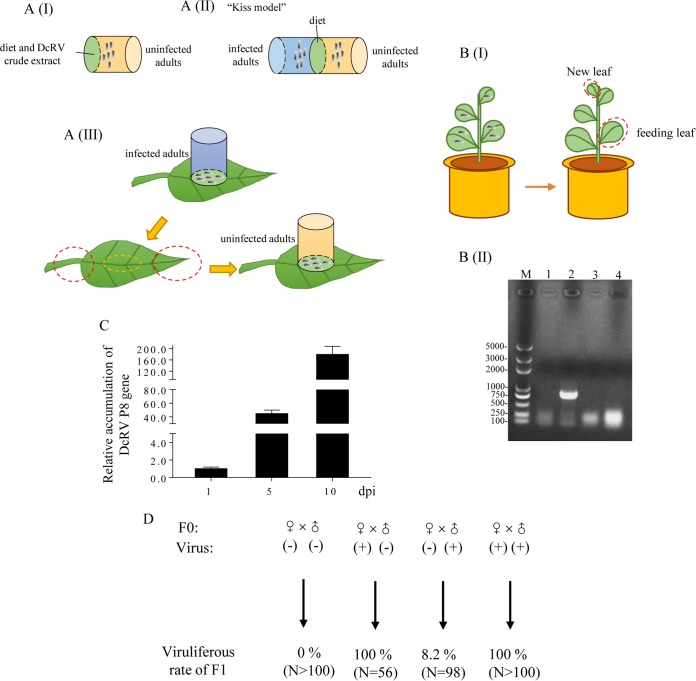
DcRV can be vertically transmitted by D. citri. (A) Schematic illustration of the feeding systems. (I) Conventional membrane feeding with diet mixed with DcRV crude extracts. (II) Kiss model for DcRV delivery and acquisition via the liquid diet. DcRV-infected and uninfected D. citri adults were confined in two individual cages, between which the diet was sandwiched with a Parafilm membrane. (III) Leaf feeding assay for DcRV delivery via the plant. DcRV-infected D. citri adults were confined in pipe-like cages and fed on a clean leaf for 8 days. Uninfected D. citri adults were fed on the same feeding zone as infected insects for 10 days. The yellow dotted circle indicates the feeding zone, and the red dotted circle indicates the distal part. (B) DcRV could not infect the orange jessamine plants. (I) Schematic illustration of the preparation for tested leaves. (II) RT-PCR analyses showing the absence of DcRV infection in orange jessamine plants. Total RNA of leaves were extracted and tested for the presence of a partial region of DcRV S8 using PCR. Lane M, DNA ladder; lane 1, leaves of healthy control plants; lane 2, DcRV-infected D. citri; lane 3, feeding leaves; lane 4, new leaves on the flush shoots of the plant that was fed on by DcRV-infected D. citri. (C) The relative accumulation of P8 RNA in organs of DcRV-injected California D. citri insects was detected using RT-qPCR assays. The results were normalized against the level of actin mRNA, and the accumulation level of P8 at 1 dpi was normalized as 1. Means (±SD) from 3 biological replicates are shown. (D) Vertical transmission of DcRV to offspring. In the parent D. citri insect, (−) means uninfected Californian insects and (+) means DcRV-infected Hawaiian insects. N is the sample size for each treatment. Data displayed here are representative of 3 replicates.