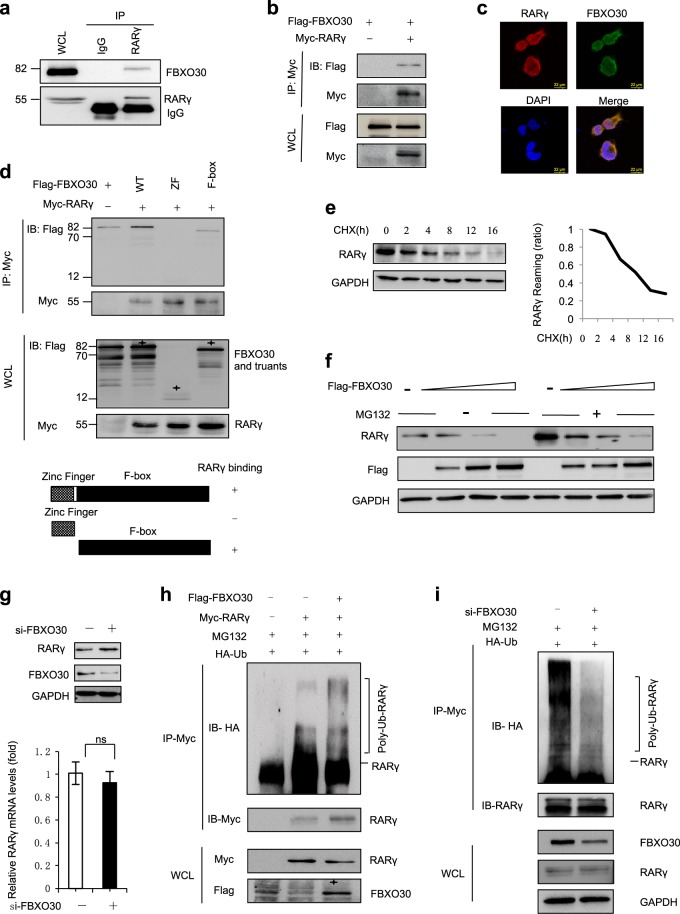
Fig. 2. FBXO30 ubiquitin ligase interacts with and ubiquitylates RARγ.
a The whole HEK293 cell extract were immunoprecipitated with anti-RARγ antibody and Normal rabbit IgG antibody, and analyzed by western blot with anti-FBXO30 and anti-RARγ antibodies. b HEK293 cells were transfected with Myc-RARγ and Flag-FBXO30 as indicated. Equal amounts of cell extract were immunoprecipitated with anti-Myc antibody, and analyzed by western blot with anti-Flag and anti-Myc antibodies. c The colocalization of FBXO30 and RARγ in the HEK293 cells. Direct immunofluorescence analysis was performed. Images were captured by confocal microscope and the nuclei were stained with DAPI. Scale bars, 22 μm. d Mapping the interacting regions of RARγ with FBXO30. HEK 293 cells were transfected with Myc-RARγ and Flag-tagged FBXO30 truncates as indicated. The cell lysates were immunoprecipitated with anti-Myc antibody and the IP samples were analyzed by western blot with Flag and Myc antibodies. The asterisks indicate the FBXO30 mutants. e HEK293 cells were treated with cycloheximide (50 μg/mL) for the indicated times and then harvested for western blotting. f HEK293 cells were transfected with increasing amounts of FBXO30. After 36 h, cells were treated with MG132 (20 μM) 12 h. Aliquots of total lysates were immunoblotted to detect anti-RARγ and anti-Flag antibody. g HEK293 cells were transfected with either control siRNA or siRNA targeting FBXO30. Endogenous FBXO30 and RARγ expression was analyzed by western blot (upper). Total RNA was subjected to real-time RT-PCR analysis (lower). Expression levels of FBXO30 and RARγ were determined by the comparative threshold cycle method. Mean values and SD are depicted (n = 3). h FBXO30 and RARγ were expressed in HEK293 cells together with HA-ubiquitin as indicated. The cells were treated with MG132 (20 μM) for 12 h. Myc-RARγ proteins were immunoprecipitated, followed by western blot with anti-HA antibody to indicate poly-ubiquitylated RARγ. i HEK293 cells were transfected with HA-Ub and siFBXO30 for 36 h and then treated with MG132 (20 μM) for 12 h. RARγ proteins were immunoprecipitated, followed by western blot with anti-HA antibody to indicate poly-ubiquitylated RARγ