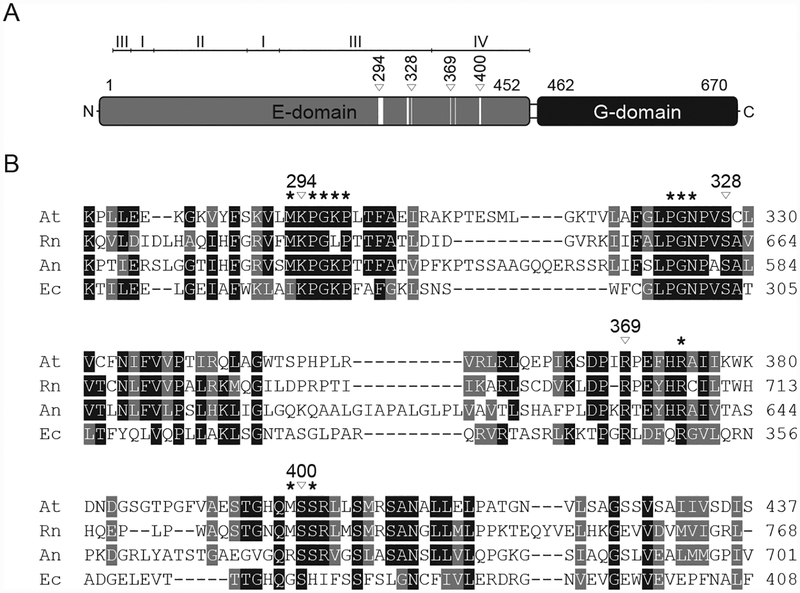
Figure 3: Cnx1 domain organization and sequence comparison of Mo-insertases from various species.
(A) Schematic representation of the Arabidopsis thaliana Cnx1 enzyme domain structure [20]. The first and last residues of the domains are indicated. For Cnx1E subdomains are indicated. Within the schematic representation of the Cnx1E domain, white boxes indicate residues forming the molybdate binding sites. From these, four are involved in directed side-chain molybdate interactions, indicated by white triangles with the corresponding Cnx1 amino acid positions given above. (B) Partial sequence comparison of Arabidopsis thaliana (At), Rattus norvegicus (Rn), Aspergillus nidulans (An) and Escherichia coli (Ec) Cnx1E homologs. Residues forming the molybdate binding site are indicated by asterisks. Cnx1 residues involved in directed molybdate interactions through their side-chains are indicated by white triangles with the corresponding Cnx1 amino acid positions given above. Strictly conserved residues are highlighted in black, conserved residues are highlighted in grey. The alignment was generated with Clustal Omega.