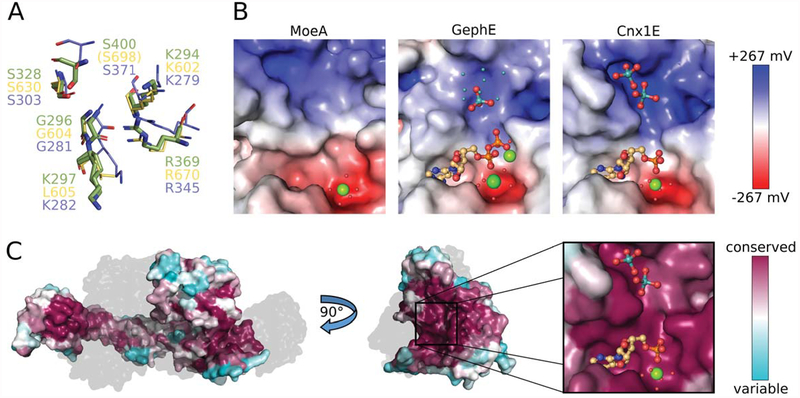
Figure 4: Properties of the oxo-anion binding pocket.
(A) Superposition of Cnx1E molybdate interacting residues with equivalent residues from GephE (Rattus norvegicus, [21]) and MoeA (Escherichia coli, [50]). Residues from Cnx1E are shown as sticks with the carbon atoms colored green. GephE and MoeA residues are shown in line representation with their carbon atoms yellow and light-blue, respectively. The residue labels are in the corresponding colors. In GephE, the amino acid residue Ser698 (corresponding to Cnx1E Ser400) is located too far away from the binding site to be displayed (see text). (B) Electrostatic properties of the molybdate binding pocket from MoeA (left, PDB 1FC5), GephE (middle, PDB 5ERU) and Cnx1E (right). The Connolly surfaces are colored according to their electrostatic potentials ranging from −267 mV(red) to +267 mV (blue). Potentials were calculated with APBS [36] for 310 K. AMP/ADP molecules and molybdate anions are shown as sticks. Mg2+ ions and water molecules are shown as big and small spheres, respectively. The small cyan-colored spheres in the GephE structure correspond to the partially occupied molybdate sites, identified by the authors only by the anomalous signal arising from molybdenum atoms [21]. The subpanels of Fig. 4C present the same view of the active site as the close-up in Fig. 4B. (C) Connolly surface of Cnx1E colored according to the degree of amino acid conservation with magenta corresponding to a high, cyan to a low degree of conservation. The conservation was calculated from a sequence alignment of Cnx1E with 27 homologous proteins from eukaryotes (see also supplementary Fig. S2). On the left, the physiological dimer of Cnx1E is depicted with the second monomer shown gray and transparent for better visibility of the dimer interface. The highest degree of conservation is found in the Cnx1E active site, which is revealed by a rotation by 90°. The active site comprises the oxo-anion and AMP binding pockets, which are shown in close-up. Molybdate and AMP are shown in ball-and-stick representation the single magnesium ion is shown as sphere. The color scheme for hetero-atoms is the same as in Fig. 2.