Abstract
Objective:
After traumatic brain injury (TBI), continuous electroencephalography (cEEG) is widely used to detect electrographic seizures (ESz). With the development of standardized cEEG terminology, we aimed to describe the prevalence and burden of ictal-interictal patterns and ESz after moderate-to-severe TBI and to correlate cEEG features with functional outcome.
Design:
Post-hoc analysis of the prospective, randomized controlled phase 2 multicenter INTREPID2566 study (ClinicalTrials.gov: NCT00805818). cEEG was initiated upon admission to the ICU. The primary outcome was the 3-month Glasgow Outcome Scale-Extended (GOSE). Consensus EEG reviews were performed by raters certified in standardized cEEG terminology blinded to clinical data. Rhythmic, periodic, or ictal patterns were referred to as ictal-interictal continuum (IIC); severe IIC was defined as ≥1.5 Hz lateralized rhythmic delta activity or generalized periodic discharges, and any lateralized periodic discharges or ESz.
Setting:
20 US Level I trauma centers
Patients:
Patients with non-penetrating TBI and post-resuscitation GCS 4–12 were included.
Interventions:
None.
Measurements and Main Results:
Among 152 patients with cEEG (age 34 ± 14 years; 88% male), 22 (14%) had severe IIC including ESz in 4 (2.6%). Severe IIC correlated with initial prognostic score (International Mission for Prognosis and Analysis of Clinical Trials in TBI (IMPACT)) (r=0.51, p=0.01) and Injury Severity Score (ISS) (r=0.49, p=0.01), but not with functional outcome. After controlling clinical covariates, unfavorable outcome was independently associated with: absence of posterior dominant rhythm (common odds ratio 3.38; 95% CI 1.30–9.09), absence of N2 sleep transients (3.69; 1.69–8.20), predominant delta activity (2.82; 1.32–6.10) and discontinuous background (5.33; 2.28–12.96).
Conclusions:
Severe IIC patterns, including ESz, were associated with clinical markers of injury severity but not functional outcome in this prospective cohort of patients with moderate-to-severe TBI. Importantly, cEEG background features were independently associated with functional outcome and improved the area-under-the-curve of existing, validated predictive models.
Keywords: Traumatic brain injury, continuous electroencephalography, neurocritical care, electrographic seizures, neurotrauma, periodic discharges, ictal-interictal continuum
INTRODUCTION
Traumatic brain injury (TBI) is a leading cause of disability and death worldwide.1 After moderate-to-severe TBI, neurological outcome prediction is critical to clinicians and families. Prognostic models employ admission clinical assessments such as the Glasgow Coma Scale (GCS), pupillary reactivity, and computed tomography (CT) findings to predict long-term outcome.2–5 The International Mission for Prognosis and Analysis of Clinical Trials in TBI (IMPACT) model was validated across more than 15,000 patients, and exhibits an area-under-the-curve between 0.66–0.84.4 However, predictive models such as IMPACT do not include information regarding brain function nor provide a dynamic assessment over time, precluding assessment of the impact of secondary brain injuries.
Continuous EEG monitoring (cEEG) has been recommended for patients with acute brain injury and altered mental status to detect electrographic seizures (ESz).6 ESz have been reported in 18–33% of adult patients with moderate-to-severe TBI6 and are associated with secondary brain injury, which may impact outcome.7–10 However, some periodic or rhythmic patterns may not meet agreed-upon definitions of ESz and the terminology to describe these patterns, which lie along an ictal-interictal continuum (IIC), has been recently standardized.11 There is little prospective data leveraging cEEG to identify ESz, and the incidence of IIC patterns have not been quantified in adults following TBI. Further, a small number of studies describing background reactivity,12 variability,13 and the presence of sleep architecture14–17 have suggested that EEG data contains prognostic information following TBI but the importance of early cEEG information in the context of established clinical predictors is unknown.
We performed a post-hoc analysis of a prospective, multicenter randomized clinical study of moderate-to-severe TBI with two objectives: (1) to assess the prevalence, characteristics, and predictors of abnormal cEEG features including ESz and (2) to determine whether specific cEEG features were predictive of 3-month functional outcome after controlling for clinical variables.
METHODS
Subjects
We performed a post-hoc analysis of the INTREPID2566 study (ClinicalTrials.gov: NCT00805818), a multicenter randomized, controlled phase 2 clinical trial conducted April 2010 to January 2016 at 20 US centers to evaluate the safety and efficacy of glycyl-L-2-methylprolyl-L-glutamic acid (NNZ-2566) in patients with moderate-to-severe TBI. NNZ-2566, a synthetic analog of a naturally-occurring neurotrophic peptide derived from insulin-like growth factor-1, demonstrated neuroprotective efficacy and reduced injury-induced seizures in rat models.18 INTREPID2566 failed to demonstrate significant differences in either adverse events or global outcome, measured by the Glasgow Outcome Scale-Extended (GOSE). Study participants included those aged 18–70 years with non-penetrating TBI, post-resuscitation GCS 4–12, post-resuscitation hemodynamic stability, and able to randomize to study drug or placebo within 8 hours of injury. Exclusion criteria were spinal cord injury, significant bodily co-injuries, prior brain injury requiring hospitalization, severe comorbidities, weight >150kg, fluid resuscitation >6L prior to randomization, and those at-risk for QT prolongation.
The INTREPID2566 study was approved by the Institutional Review Board at each participating institution. Written informated consent was obtained from all patients or their legal surrogates.
Data Collection
The trial protocol included bedside cEEG initiated upon admission to the ICU and continued at least through the 72-hr maintenance drug infusion. cEEG was not performed in some patients who required immediate neurosurgical intervention, whose family members withdrew life-sustaining treatments, or who were admitted while sites were not yet validated for cEEG. Thirteen scalp electrodes were positioned based on the International 10–20 System: Fp1, F7, C3, T3, T5, O1, Cz, Fp2, F8, C4, T4, T6, and O2. Clinical features were collected including demographics, TBI-specific parameters (i.e., injury description, Injury Severity Score (ISS), CT classification within the first 24 hours), neurological examination findings (i.e., post-resuscitation GCS, pupillary reactivity), the use of sedative agents and anti-seizure drugs (ASDs), and 3-month GOSE, an eight-point ordinal functional outcome scale.
Electroencephalographic Review
cEEG analysis was performed by three raters (H.L., B.F., and M.M.) blinded to patient clinical information and interpreted every 4 hours for the first 24 hours and daily for up to 4 subsequent days. Raters were certified in the ACNS’ Standardized Critical Care EEG Terminology.11 Certification consists of review of training modules available on the ACNS website (https://www.acns.org/research/critical-care-eeg-monitoring-research-consortium-ccemrc) followed by interpretation of standardized EEG samples via an online test. Standardized terminology was used to describe IIC patterns and dominant background frequency, posterior dominant rhythm (PDR), background fast activity, and discontinuity (simplified as >10% of the record consisting of attenuation or suppression). Sleep was stratified as the presence of normal (based on published criteria21) or rudimentary N2 sleep transients (K complexes and/or sleep spindles), or their absence.17 Background EEG classifications are summarized in Table 1 and Figure 1.19, 22 IIC patterns were classified according to their potential association with seizure activity or neuronal injury according to recent evidence (Table 1 and Figure 2).19 ESz were defined as any spikes, sharp waves, or sharp-and-slow wave complexes with a frequency >2.5 Hz lasting for 10s, or any rhythmic or periodic discharges (i.e. IIC patterns) with clear evolution in frequency, morphology, or location.20 Severe IIC was defined as ≥1.5 Hz lateralized rhythmic delta activity (LRDA) or generalized periodic discharges (GPD), and any lateralized periodic discharges (LPD) or ESz. Finally, we calculated the IIC burden based on IIC prevalence and frequency (e.g., if 1.5-Hz periodic discharges were detected for 50% of an 8-hr record, then 25% of the next 24-hr, the IIC duration would be 4-hr + 6-hr = 10-hr, and the IIC burden would be 1.5-Hz × 10-hr = 15 Hz-hours) and adjusted for recording duration.11 Because of the small sample size in the severe background and severe IIC categories, moderate and severe groups were combined for primary outcome analysis.
Table 1.
cEEG classification for analysis
| Abnormalities | Description | |
|---|---|---|
| Background | I Normal / Mild | Predominant alpha to theta activity, NREM or rudimentary sleep |
| II Moderate | Predominant delta activity and absent N2 sleep transients | |
| III Severe | Burst-attenuation, burst-suppression or suppression | |
| IIC | I Normal | None |
| II Mild | GRDA | |
| III Moderate | Slower frequency (<1.5 Hz) LRDA or GPDs | |
| IV Severe | Faster frequency (≥1.5 Hz) LRDA or GPDs, LPDs or ESz |
cEEG, continuous electroencephalography; NREM, non-rapid eye movement; IIC, ictal-interictal continuum; GRDA, generalized rhythmic delta activity; LRDA, lateralized rhythmic delta activity; GPDs, generalized periodic discharges; LPDs, lateralized periodic discharges; ESz, electrographic seizure.
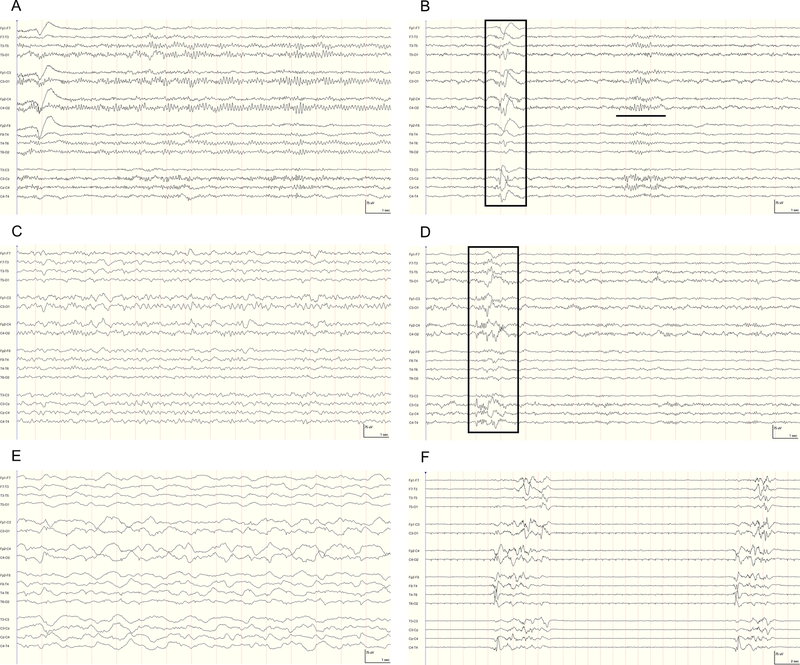
Figure 1.
Representative continuous electroencephalography (cEEG) epoch demonstrating background classification. High-frequency filter=70 Hz, notch filter=60 Hz, and time constant=0.12 s. (A) I Normal: 9-Hz posterior dominant (alpha) rhythm during a period of relative wakefulness. (B) I Normal: N2 sleep with a prominent sleep spindle (bold line) and K-complex (box). (C) I Mild: predominant theta background activity. (D) I Mild: rudimentary N2 sleep transients (K-complex, box). (E) II Moderate: continuous, irregular 1 to 3-Hz delta activity without N2 sleep transients. (F) III Severe: bursts (>500ms and >3phases) of generalized activity on a suppressed (<10 μV) background, or burst-suppression pattern.
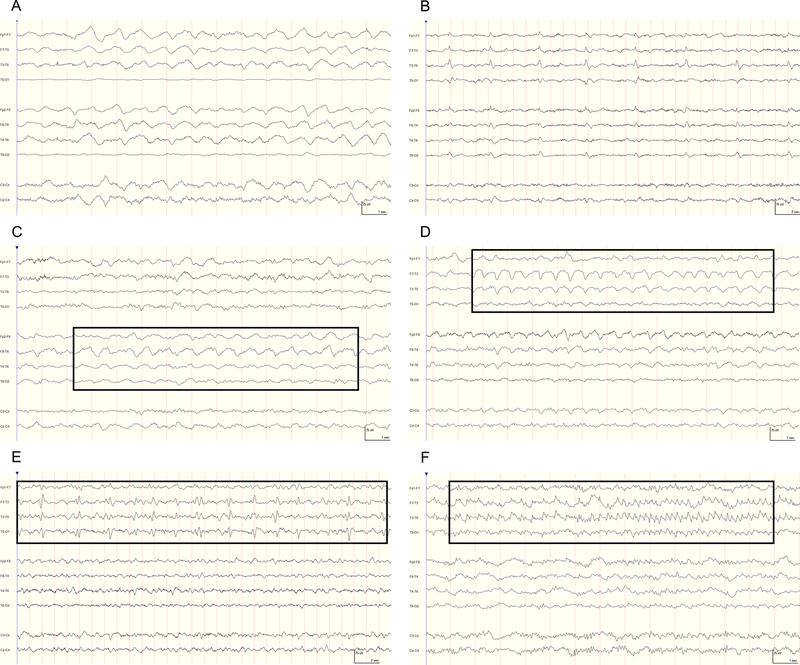
Figure 2.
Representative continuous electroencephalography (cEEG) epochs demonstrating ictal-interictal continuum (IIC) classification. High-frequency filter=70 Hz, notch filter=60 Hz, and time constant=0.12 s. (A) IIC II Mild: generalized rhythmic delta activity (GRDA) that is frontally predominant with a frequency of approximately 1-Hz. (B) III Moderate: slow-frequency (0.25-Hz) generalized periodic discharges (GPDs). (C) III Moderate: lateralized rhythmic delta activity (LRDA) with a frequency of approximately 1-Hz (box), most prominent in the right temporal region. (D) IV Severe: LRDA between 1.5 to 2-Hz (box), predominantly in the left temporal region. (E) IV Severe: irregular (in morphology and repetition rate) 0.5-Hz lateralized periodic discharges (LPDs) in the left hemisphere. (F) IV Severe: electrographic seizure (ESz) that evolves in morphology and frequency from 2-Hz to 5-Hz rhythmic discharges in the left temporal region.
Statistical Analysis
Data were analyzed using R (version 3.4.3: R Core Team, 2017) and GraphPad Prism (version 7.03: GraphPad Software, 2017). Statistical data were summarized as mean±standard deviation or median (interquartile range [IQR]) where appropriate. Functional outcome was dichotomized as unfavorable (GOSE 1–4; death, unresponsive wakefulness, or severe disability) or favorable (GOSE 5–8; moderate disability or good recovery). Univariate analysis was conducted using χ2 or Fisher’s exact tests and independent sample t-tests as appropriate. Correlation between continuous variables was assessed using Pearson’s correlation coefficient. All tests were two-sided with significance considered at p<0.02 to account for multiple comparisons. A proportional odds logistic regression model was constructed to evaluate independent predictors of the ordinal 3-month GOSE including the IMPACT core linear predictor (LP), calculated using age, GCS motor score, and pupillary reactivity, in order to leverage the larger sample size used to derive this predictor.4, 23 Significant cEEG variables associated with unfavorable outcome were individually added to the model to generate common odds ratios (OR) and 95% confidence intervals (CI). Study drug treatment allocation was incorporated to exclude the impact of study drug. cEEG features were assessed for sensitivity, specificity and area-under-the-curve (AUC) based on receiver operating characteristic curves and compared to the IMPACT core model.
RESULTS
Patient Characteristics
Overall, 261 patients with moderate-to-severe TBI were enrolled in the INTREPID2566 study. cEEG was performed in 155/261 (59%) patients. Table 2 shows the overall patient characteristics and differences between those undergoing cEEG vs. those without cEEG. Of those undergoing cEEG, three patients were excluded based on inadequate cEEG quality, yielding 152 patients with 10,405 hours of cEEG for this study. The mean age of these 152 patients was 34.5±14.8 years, and 134/152 (88%) were male. The median admission GCS was 7 [6–8] and the median ISS was 24 [14–30]. Admission CT demonstrated subarachnoid hemorrhage (SAH) in 108/152 (71%), intraventricular hemorrhage in 49/152 (32%), a mass lesion in 39/152 (26%), midline shift in 39/152 (26%), and cisternal compression in 36/152 (24%). 111/152 (73%) patients received GABAergic sedatives (e.g. propofol, pentobarbital, midazolam, lorazepam or diazepam) and 134/152 (88%) received an ASD starting a median of 1 [0–2] days after injury, maintained for 6 [5–8] days. The median duration of cEEG monitoring was 70 [42–101] hours starting a median 10 [8–14] hours after injury.
Table 2.
Patient Demographics
| Variable | Complete Cohort (n=261) | cEEG (n=155) | No cEEG (n=106) | p-value |
|---|---|---|---|---|
| Age | 34.4+/−14.5 | 34.5+/−14.9 | 34.4+/−13.9 | 0.95 |
| Gender (male) | 230 (88.1) | 137 (88.4) | 93 (88.6) | 1.00 |
| Total Glasgow Coma Scale Score | 7 (6–9) | 7 (6–8) | 7 (6–9) | 0.83 |
| Pupils, ≥1 unreactive* | 22 (8.4) | 18 (11.6) | 4 (3.8) | 0.04 |
| Injury severity score | 24 (14–33) | 24 (15–30) | 26 (14–34) | 0.31 |
| IMPACT sum score | 3.1+/−2.6 | 3.0+/−2.6 | 3.3+/−2.7 | 0.51 |
| Marshall CT Class | 0.01 | |||
| I (normal CT) | 30/257 (11.7) | 12 (7.7) | 18/102 (17.6) | |
| II (cisterns present, shift<5mm) | 87/257 (33.9) | 59 (38.1) | 28 (27.5) | |
| III (cisters compressed, shift <5mm) | 45/257 (17.5) | 31 (20.0) | 14 (13.7) | |
| IV (shift>5mm) | 29/257 (11.3) | 14 (9.0) | 15 (14.7) | |
| V (evacuated mass) | 20/257 (7.8) | 10 (6.5) | 10 (9.8) | |
| VI (nonevacuated mass) | 46/257 (17.9) | 29 (18.7) | 17 (16.7) | |
| Presence of traumatic SAH | 164/257 (63.8) | 111 (71.6) | 53 (52) | <0.01 |
| Presence of traumatic IVH | 72/257 (28.0) | 51 (32.9) | 21 (20.6) | 0.05 |
| Study drug (vs. placebo) | 167 (64.0) | 102 (65.8) | 65 (61.3) | 0.54 |
| Antiseizure drug administration | 216 (82.8) | 137 (88.4) | 79 (74.5) | 0.01 |
| Sedation with GABA agonist | 167 (64.0) | 112 (72.3) | 55 (51.9) | <0.01 |
| Outcome (poor, GOS 1–4)** | 114/220 (51.8) | 73/142 (51.4) | 41/78 (52.6) | 0.98 |
Only a single patient had bilaterally nonreactive pupils in the group undergoing cEEG.
Follow up at 3 months was available for 220/261 (84.3%) of patients
cEEG Findings
IIC patterns were observed in 74/152 (49%) patients. LPDs, GPDs and LRDA patterns were recorded in 9/152 (5.9%), 8/152 (5.2%), and 18/152 (12%) of patients, respectively. Severe IIC was seen in 22/152 (14%) patients, of whom four (2.6%) had ESz. Severe IIC occurred ≤4 hours of cEEG initiation in 7/22 (32%); ≤48 hours in 8/22 (36%); and >48 hours of cEEG in the remaining 7/22 (32%). Patients with and without severe IIC on cEEG had similar age, GCS, pupillary exam, and CT findings; there was no difference in the use of ASDs or sedatives initiated within 48 hours post-injury in those with and without severe IIC (13/22 [59%] vs. 99/130 [76%] for ASDs, p=0.15; 11/22 [50%] vs. 72/130 [55%] for GABAergic sedatives, p=0.81). The burden of severe IIC patterns correlated significantly with IMPACT score (r=0.51, p=0.01) and ISS (r=0.49, p=0.01; Supplemental Figure 1). Moderate-to-severe background activity was observed in more than half of patients (90/152; 59%) and the majority (135/152; 89%) had superimposed background fast activity. A PDR was observed in 31/152 (20%) patients, and 43/152 (28%) patients had either well-structured or rudimentary N2 sleep transients.
cEEG Features and Functional Outcome
Overall, 139/152 (91%) patients had 3-month GOSE, of whom 71/139 (51%) had unfavorable outcome. Unfavorable outcome was not associated with age or pupillary reactivity in this cohort, but was associated with post-resuscitation GCS and ISS (Supplemental Table 1). There was no association between the presence of moderate-to-severe IIC, including ESz, and functional outcome. There was similarly no relationship between the burden of IIC categories and dichotomized outcomes.
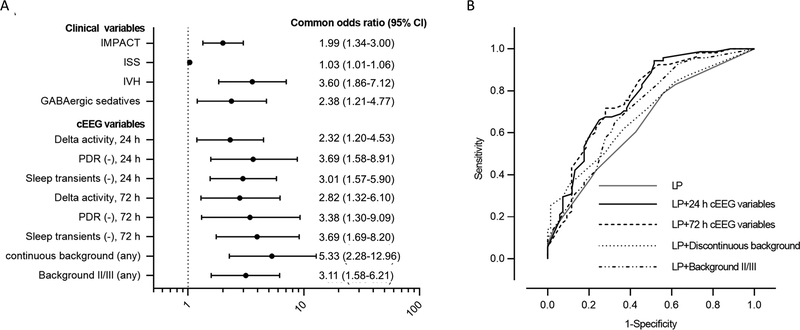
Multivariate analysis demonstrated an independent association between unfavorable outcome and the absence of PDR (OR 3.38; 95% CI 1.30–9.09), the absence of N2 sleep transients (OR 3.69; 95% CI 1.69–8.20), the presence of predominant background delta activity (OR 2.82; 95% CI 1.32–6.10), a discontinuous background at any time during recording (OR 5.33; 95% CI 2.28–12.96) and the presence of moderate-to-severe background at any time of recording (OR 3.11; 95% CI 1.58–6.21) during the first 72 hours of cEEG recording after adjusting for IMPACT clinical core model, ISS, IVH, GABAergic sedative administration and study drug allocation (Figure 3; detailed results in Supplemental Statistical Table).
Figure 3.
Forest plot and receiver operator characteristic curves (ROC) of continuous electroencephalography (cEEG) variables associated with an increased risk of unfavorable outcome. (A) Common odds ratios are adjusted for International Mission for Prognosis and Analysis of Clinical Trials in traumatic brain injury (IMPACT) score (age, Glasgow Coma Scale motor score, pupillary reactivity), Injury Severity Score (ISS), intraventricular hemorrhage (IVH), GABAergic sedatives and infusion of study drug and are displayed on a logarithmic scale. (B) ROC showing accuracy for prediction of dichotomized unfavorable outcome by linear predictor (LP) from IMPACT core model and LP combined with cEEG variables. The area-under-the-curve (AUC) values were 0.649 for the LP, 0.768 after adding 24-hr cEEG variables to the LP, 0.764 after adding 72-hr cEEG variables to LP, 0.689 after adding discontinuous background to LP and 0.712 after adding moderate-to-severe background to LP. CI, confidence interval; PDR, posterior dominant rhythm.
The sensitivity and specificity of the IMPACT covariates alone to predict unfavorable outcome were 79% and 44%, respectively, with an AUC of 0.649. Adding three features (absence of PDR, absence of N2 sleep transients, and predominant background delta activity) during the first 24 hours of cEEG to the IMPACT covariates resulted in a sensitivity and specificity for unfavorable outcome of 94% and 49%, with an AUC of 0.768. The presence of these features (a PDR, N2 sleep transients, and predominantly theta or alpha background) within 72 hours predicted good outcome with a specificity of 96% and positive predictive value of 86%, but only 28% sensitivity.
DISCUSSION
In this post-hoc analysis of a randomized, controlled trial of patients with moderate-to-severe TBI, we found severe IIC patterns, including ESz, in 14%. The burden of these patterns was associated with disease severity but not functional outcome at 3 months. We determined that the cEEG background characteristics including PDR, N2 sleep transients, predominant background frequency and continuity were independently associated with functional outcome at 3 months. In our cohort, the addition of cEEG variables observed within the first 24 and 72 hours of recording increased the predictive ability of the IMPACT core predictor variables.
Ours is the largest study of patients with TBI specifically focusing on scalp cEEG interpreted using ACNS’ Standardized Critical Care EEG Terminology. The prevalence of both LPDs (9/152; 5.9%) and GPDs (8/152; 5.2%) were similar to previous reports, while LRDA was recorded in 12% (18/152) of patients, which is higher than that reported in previous studies (4.7–7.1% in general ICU population).24–26 We found fewer seizures, only 4/152 (2.6%), compared to previous reports (19–33%),7, 26, 27 which may be related to the fact that 88% of patients received ASDs28 or that cEEG was not performed in 41% of patients, many of whom were excluded due to anticipated mortality or withdrawal of life-sustaining therapy decisions. Alternatively, patterns we described as severe IIC may have been interpreted clinically as ESz in studies conducted prior to the establishment of standardized terminology. This distinction may be of questionable clinical significance since both IIC patterns and seizures can create similar physiologic abnormalities in the setting of acute brain injuries.10, 29
Despite increasing evidence to suggest an association between severe IIC, clinical seizures19, 25, 30, 31 and pathophysiologic correlates of secondary brain injury,10, 32, 33 it remains unclear whether or not these patterns have independent predictive value with regard to outcome. Periodic discharges have been shown to be an independent predictor for unfavorable outcome in some populations,29, 34 but not in others.25, 35 Our study included a specific TBI population with a small sample size of patients with severe IIC, which may have under-powered our results despite the use of a continuous estimate of severe IIC, termed the IIC burden. Other outcome endpoints, such as cognitive measurements, may be more relevant considering the potential for severe IIC to disinhibit excitatory pyramidal cells36 and increasing evidence for the impact of interictal epileptiform discharges on cognition in patients with epilepsy.37, 38 Interestingly, the burden of severe IIC was positively correlated with admission injury severity, which suggests that the clinical and pathophysiological significance of IIC patterns may be related to or modulated by underlying brain and even systemic injuries, and vice versa. This has been suggested in patients with SAH in whom inflammatory pathways have been linked with both ESz and outcome.39 Conclusions about IIC patterns in the population evaluated in this study may not apply to the general critical care population, however.
We examined the cEEG background, a reflection of underlying brain functioning, and our findings are consistent with those of previous studies examining reactivity,12 variability,13 sleep,15–17 and dominant background frequency40 after TBI. However, in most studies, predictors were investigated as isolated phenomena using retrospectively-collected data. One study suggested a weighted dichotomous score to summate individual EEG predictors (i.e., background frequency, asymmetry, reactivity, variability, and additional patterns including epileptiform activity) and found this correlated with discharge GOSE in 57 TBI patients.41 Although this numeric score provided an objective tool for EEG review, the method of weighting each parameter was arbitrary, and major clinical confounders were not controlled. We found that a moderate-to-severe background pattern at any time during cEEG was associated with unfavorable functional outcome even after controlling for established clinical variables.
Since TBI involves dynamic pathophysiology that evolves in time,42–44 we investigated whether development or loss of specific cEEG features over time associated with outcome. Interestingly, the absence of PDR for 24 hours had a higher odds ratio for unfavorable outcome than the absence of PDR for 72 hours, whereas both the absence of N2 sleep transients and delta activity exhibited increasing odds ratios over time. This may be explained by a time-dependent loss of PDR in patients with moderate-to-severe TBI or a decrease in statistical power as the number of patients monitored fell (i.e., n=139 for 24 hours vs. n=96 for 72 hours). By combining cEEG background features, we found that the addition of either 24-hr or 72-hr cEEG predictors to the core clinical IMPACT predictor model increased its AUC from 0.64, which is traditionally interpreted as poorly accurate (0.60–0.70), to 0.76, or moderately accurate (0.70–0.90).45 Therefore, information gained from cEEG as early as 24 hours after initiation might warrant incorporation into clinical prediction models.
Our study used data post-hoc from a study that was not necessarily powered to answer the questions raised by our hypothesis. We estimate that 298 subjects per group would be required to demonstrate a significant association between severe IIC burden and unfavorable outcome at 80% power using our p-value threshold. Further, only 59% (155/261) of enrolled participants underwent cEEG despite study protocol, which may have biased our sample. Patients with cEEG appeared to have more severe injury compared to those without; however, surgery and withdrawal of life-sustaining measures were reasons that patients were excluded from cEEG. Both groups had similar injury severity scores. The number of patients with cEEG decreased over time (Supplementary Figure 2), which reduced our power to make inferences about the evolution of cEEG findings and potentially led to missed ESz.26 Despite our relatively large cohort, our study sample did not allow for more precision in the categorization of background categories, sleep classification, or IIC patterns. As a result of the lack of video information and literature evidence for poor inter-rater agreement,46 we did not assess EEG reactivity or stimulus-induced rhythmic, periodic, or ictal discharges (SIRPIDs), which also may be important in predicting outcome after severe brain injury.
In conclusion, our data suggest that the cEEG background as early as 24 hours after initiation of the recording may provide additive prognostic information in the context of established clinical variables. We found that severe IIC patterns, while relatively common in patients with moderate-to-severe TBI, were not associated with functional outcome at 3 months although further studies are warranted to understand the effects of severe IIC patterns on more refined outcome measures such as cognition.
Supplementary Material
Supplemental Figure 1. Correlation between burden of ictal-interictal continuum (IIC) and clinical assessment score. (A) All IIC burden and International Mission for Prognosis and Analysis of Clinical Trials in traumatic brain injury (IMPACT) score, which is sum of the score based on age, Glasgow Coma Scale motor score, and pupillary reactivity. (B) Moderate to severe IIC burden and IMPACT. (C) Severe IIC burden and IMPACT. (D) All IIC burden and Injury Severity Score (ISS), which is an anatomical scoring system for patients with multiple injuries including head & neck, face, chest, abdomen, extremity and external. (E) Moderate to severe IIC burden and ISS. (F) Severe IIC burden and ISS.
Supplemental Figure 2. A line graph demonstrating the number of patients undergoing cEEG based on (top) the number of hours of monitoring through the 5 day period and (bottom) the number of hours from trauma.
Sources of Funding:
The INTREPID2566 trial was supported in part by grants from the U.S. Army Medical Research and Materiel Command (FT) and by Neuren Pharmaceuticals Limited. Research reported in this publication was supported by the National Institute Of Neurological Disorders And Stroke of the National Institutes of Health under Award Number K23NS101123 (BF). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Defense or the National Institutes of Health.
Potential Conflicts of Interest:
Research funding for the parent randomized controlled trial were obtained in part through the DOD by one of the study authors (FT), who served as principal investigator for the parent randomized controlled trial. Study authors (BM and MP) received research funds through Neuren Pharmaceuticals Limited. for participation in parent randomized controlled trial. The remaining study authors have nothing to report.
REFERENCES
- 1.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. The Journal of head trauma rehabilitation. 2006. Sep-Oct;21(5):375–8. [DOI] [PubMed] [Google Scholar]
- 2.Hukkelhoven CW, Steyerberg EW, Habbema JD, et al. Predicting outcome after traumatic brain injury: development and validation of a prognostic score based on admission characteristics. Journal of neurotrauma. 2005. October;22(10):1025–39. [DOI] [PubMed] [Google Scholar]
- 3.Perel P, Arango M, Clayton T, et al. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ (Clinical research ed). 2008. February 23;336(7641):425–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steyerberg EW, Mushkudiani N, Perel P, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008. August 5;5(8):e165; discussion e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Husson EC, Ribbers GM, Willemse-van Son AH, Verhagen AP, Stam HJ. Prognosis of six-month functioning after moderate to severe traumatic brain injury: a systematic review of prospective cohort studies. Journal of rehabilitation medicine. 2010. May;42(5):425–36. [DOI] [PubMed] [Google Scholar]
- 6.Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2015. April;32(2):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vespa PM, Nuwer MR, Nenov V, et al. Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. Journal of neurosurgery. 1999. November;91(5):750–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vespa PM, Miller C, McArthur D, et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Critical care medicine. 2007;35(12):2830. [PMC free article] [PubMed] [Google Scholar]
- 9.Vespa PM, McArthur DL, Xu Y, et al. Nonconvulsive seizures after traumatic brain injury are associated with hippocampal atrophy. Neurology. 2010. August 31;75(9):792–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vespa P, Tubi M, Claassen J, et al. Metabolic crisis occurs with seizures and periodic discharges after brain trauma. Annals of neurology. 2016. April;79(4):579–90. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch LJ, LaRoche SM, Gaspard N, et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2013. February;30(1):1–27. [DOI] [PubMed] [Google Scholar]
- 12.Gütling E, Gonser A, Imhof H-G, Landis T. EEG reactivity in the prognosis of severe head injury. Neurology. 1995;45(5):915–8. [DOI] [PubMed] [Google Scholar]
- 13.Bricolo A, Faccioli F, Turazzi S. [EEG in post-traumatic coma. Diagnostic and prognostic value (author’s transl)]. Revue d’electroencephalographie et de neurophysiologie clinique. 1979. Apr-Jun;9(2):116–30. [DOI] [PubMed] [Google Scholar]
- 14.Rumpl E, Prugger M, Bauer G, Gerstenbrand F, Hackl JM, Pallua A. Incidence and prognostic value of spindles in post-traumatic coma. Electroencephalography and clinical neurophysiology. 1983. November;56(5):420–9. [DOI] [PubMed] [Google Scholar]
- 15.Sandsmark DK, Kumar MA, Woodward CS, Schmitt SE, Park S, Lim MM. Sleep Features on Continuous Electroencephalography Predict Rehabilitation Outcomes After Severe Traumatic Brain Injury. The Journal of head trauma rehabilitation. 2016. Mar-Apr;31(2):101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans BM, Bartlett JR. Prediction of outcome in severe head injury based on recognition of sleep related activity in the polygraphic electroencephalogram. Journal of neurology, neurosurgery, and psychiatry. 1995. July;59(1):17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valente M, Placidi F, Oliveira AJ, et al. Sleep organization pattern as a prognostic marker at the subacute stage of post-traumatic coma. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2002. November;113(11):1798–805. [DOI] [PubMed] [Google Scholar]
- 18.Lu XC, Si Y, Williams AJ, Hartings JA, Gryder D, Tortella FC. NNZ-2566, a glypromate analog, attenuates brain ischemia-induced non-convulsive seizures in rats. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2009. December;29(12):1924–32. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez Ruiz A, Vlachy J, Lee JW, et al. Association of Periodic and Rhythmic Electroencephalographic Patterns With Seizures in Critically Ill Patients. JAMA Neurol. 2017. February 1;74(2):181–8. [DOI] [PubMed] [Google Scholar]
- 20.Leitinger M, Beniczky S, Rohracher A, et al. Salzburg Consensus Criteria for Non-Convulsive Status Epilepticus--approach to clinical application. Epilepsy Behav. 2015. August;49:158–63. [DOI] [PubMed] [Google Scholar]
- 21.Silber MH, Ancoli-Israel S, Bonnet MH, et al. The visual scoring of sleep in adults. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2007. March 15;3(2):121–31. [PubMed] [Google Scholar]
- 22.Synek VM. Prognostically important EEG coma patterns in diffuse anoxic and traumatic encephalopathies in adults. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 1988. April;5(2):161–74. [DOI] [PubMed] [Google Scholar]
- 23.Hartings JA, Bullock MR, Okonkwo DO, et al. Spreading depolarisations and outcome after traumatic brain injury: a prospective observational study. The Lancet Neurology. 2011. December;10(12):1058–64. [DOI] [PubMed] [Google Scholar]
- 24.Lee JW, LaRoche S, Choi H, et al. Development and Feasibility Testing of a Critical Care EEG Monitoring Database for Standardized Clinical Reporting and Multicenter Collaborative Research. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2016. April;33(2):133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foreman B, Claassen J, Abou Khaled K, et al. Generalized periodic discharges in the critically ill. A case-control study of 200 patients. 2012;79(19):1951–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004. May 25;62(10):1743–8. [DOI] [PubMed] [Google Scholar]
- 27.Ronne‐Engstrom E, Winkler T. Continuous EEG monitoring in patients with traumatic brain injury reveals a high incidence of epileptiform activity. Acta Neurologica Scandinavica. 2006;114(1):47–53. [DOI] [PubMed] [Google Scholar]
- 28.Liesemer K, Bratton SL, Zebrack CM, Brockmeyer D, Statler KD. Early post-traumatic seizures in moderate to severe pediatric traumatic brain injury: rates, risk factors, and clinical features. Journal of neurotrauma. 2011. May;28(5):755–62. [DOI] [PubMed] [Google Scholar]
- 29.Claassen J, Hirsch LJ, Frontera JA, et al. Prognostic significance of continuous EEG monitoring in patients with poor-grade subarachnoid hemorrhage. Neurocritical care. 2006;4(2):103–12. [DOI] [PubMed] [Google Scholar]
- 30.Gaspard N, Manganas L, Rampal N, Petroff OA, Hirsch LJ. Similarity of lateralized rhythmic delta activity to periodic lateralized epileptiform discharges in critically ill patients. JAMA Neurol. 2013. October;70(10):1288–95. [DOI] [PubMed] [Google Scholar]
- 31.Struck AF, Ustun B, Ruiz A, et al. Association of an electroencephalography-based risk score with seizure probability in hospitalized patients. JAMA Neurology. 2017;74(12):1419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Witsch J, Frey HP, Schmidt JM, et al. Electroencephalographic Periodic Discharges and Frequency-Dependent Brain Tissue Hypoxia in Acute Brain Injury. JAMA Neurol. 2017. March 01;74(3):301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Struck AF, Westover MB, Hall LT, Deck GM, Cole AJ, Rosenthal ES. Metabolic Correlates of the Ictal-Interictal Continuum: FDG-PET During Continuous EEG. Neurocritical care. 2016. June;24(3):324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Claassen J, Jette N, Chum F, et al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology. 2007. September 25;69(13):1356–65. [DOI] [PubMed] [Google Scholar]
- 35.Crepeau AZ, Kerrigan JF, Gerber P, et al. Rhythmical and periodic EEG patterns do not predict short-term outcome in critically ill patients with subarachnoid hemorrhage. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2013. June;30(3):247–54. [DOI] [PubMed] [Google Scholar]
- 36.van Putten MJ, Hofmeijer J. Generalized periodic discharges: Pathophysiology and clinical considerations. Epilepsy Behav. 2015. August;49:228–33. [DOI] [PubMed] [Google Scholar]
- 37.Lv Y, Wang Z, Cui L, Ma D, Meng H. Cognitive correlates of interictal epileptiform discharges in adult patients with epilepsy in China. Epilepsy Behav. 2013. October;29(1):205–10. [DOI] [PubMed] [Google Scholar]
- 38.Ebus S, Arends J, Hendriksen J, et al. Cognitive effects of interictal epileptiform discharges in children. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society. 2012. November;16(6):697–706. [DOI] [PubMed] [Google Scholar]
- 39.Claassen J, Albers D, Schmidt JM, et al. Nonconvulsive seizures in subarachnoid hemorrhage link inflammation and outcome. Annals of neurology. 2014. May;75(5):771–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beridze M, Khaburzania M, Shakarishvili R, Kazaishvili D. Dominated EEG patterns and their prognostic value in coma caused by traumatic brain injury. Georgian medical news. 2010. September(186):28–32. [PubMed] [Google Scholar]
- 41.Rae-Grant AD, Barbour PJ, Reed J. Development of a novel EEG rating scale for head injury using dichotomous variables. Electroencephalography and clinical neurophysiology. 1991. November;79(5):349–57. [DOI] [PubMed] [Google Scholar]
- 42.Hinzman JM, Andaluz N, Shutter LA, et al. Inverse neurovascular coupling to cortical spreading depolarizations in severe brain trauma. Brain : a journal of neurology. 2014. November;137(Pt 11):2960–72. [DOI] [PubMed] [Google Scholar]
- 43.Thelin EP, Tajsic T, Zeiler FA, et al. Monitoring the Neuroinflammatory Response Following Acute Brain Injury. Frontiers in Neurology. 2017 2017-July-20;8(351). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. British journal of anaesthesia. 2007. July;99(1):4–9. [DOI] [PubMed] [Google Scholar]
- 45.Swets JA. Measuring the accuracy of diagnostic systems. Science (New York, NY). 1988. June 3;240(4857):1285–93. [DOI] [PubMed] [Google Scholar]
- 46.Alsherbini KA, Plancher JM, Ficker DM, et al. Stimulus-Induced Rhythmic, Periodic, or Ictal Discharges in Coma-Incidence and Interrater Reliability of Continuous EEG After a Standard Stimulation Protocol: A Prospective Study. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2017. July;34(4):375–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Correlation between burden of ictal-interictal continuum (IIC) and clinical assessment score. (A) All IIC burden and International Mission for Prognosis and Analysis of Clinical Trials in traumatic brain injury (IMPACT) score, which is sum of the score based on age, Glasgow Coma Scale motor score, and pupillary reactivity. (B) Moderate to severe IIC burden and IMPACT. (C) Severe IIC burden and IMPACT. (D) All IIC burden and Injury Severity Score (ISS), which is an anatomical scoring system for patients with multiple injuries including head & neck, face, chest, abdomen, extremity and external. (E) Moderate to severe IIC burden and ISS. (F) Severe IIC burden and ISS.
Supplemental Figure 2. A line graph demonstrating the number of patients undergoing cEEG based on (top) the number of hours of monitoring through the 5 day period and (bottom) the number of hours from trauma.