Abstract
Importance
Relapse prevention in recurrent depression is a significant public health problem, and antidepressants are the current first-line treatment approach. Identifying an equally efficacious nonpharmacological intervention would be an important development.
Objective
To conduct a meta-analysis on individual patient data to examine the efficacy of mindfulness-based cognitive therapy (MBCT) compared with usual care and other active treatments, including antidepressants, in treating those with recurrent depression.
Data Sources
English-language studies published or accepted for publication in peer-reviewed journals identified from EMBASE, PubMed/Medline, PsycINFO, Web of Science, Scopus, and the Cochrane Controlled Trials Register from the first available year to November 22, 2014. Searches were conducted from November 2010 to November 2014.
Study Selection
Randomized trials of manualized MBCT for relapse prevention in recurrent depression in full or partial remission that compared MBCT with at least 1 non-MBCT treatment, including usual care.
Data Extraction and Synthesis
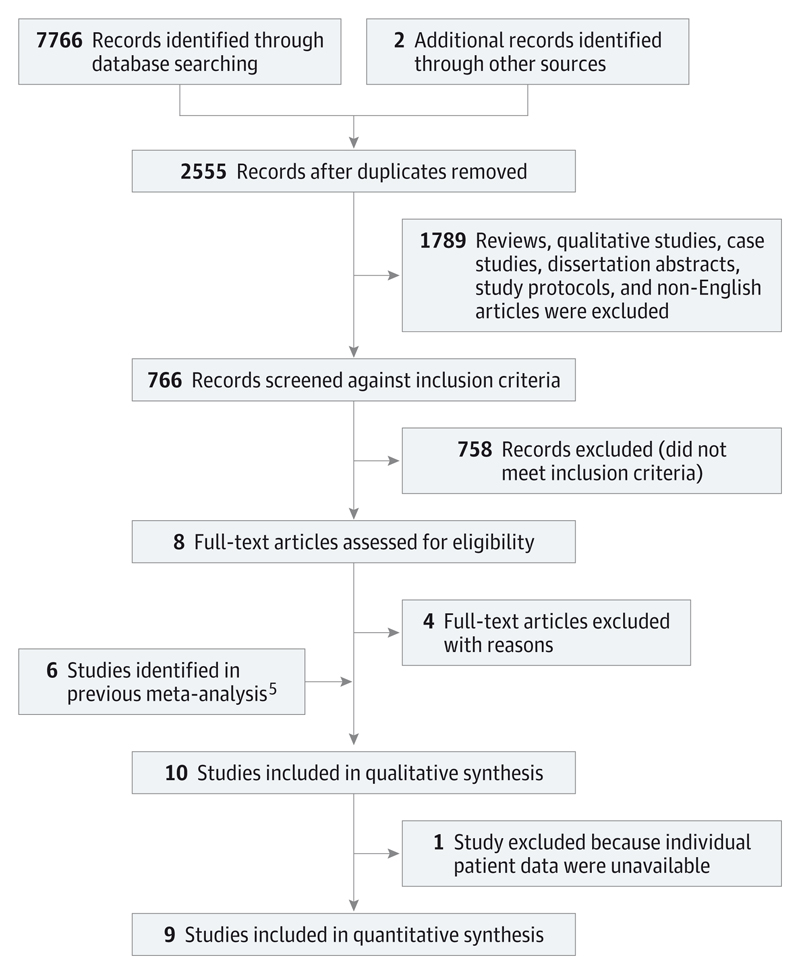
This was an update to a previous meta-analysis. We screened 2555 new records after removing duplicates. Abstracts were screened for full-text extraction (S.S.) and checked by another researcher (T.D.). There were no disagreements. Of the original 2555 studies, 766 were evaluated against full study inclusion criteria, and we acquired full text for 8. Of these, 4 studies were excluded, and the remaining 4 were combined with the 6 studies identified from the previous meta-analysis, yielding 10 studies for qualitative synthesis. Full patient data were not available for 1 of these studies, resulting in 9 studies with individual patient data, which were included in the quantitative synthesis.
Results
Of the 1258 patients included, the mean (SD) age was 47.1 (11.9) years, and 944 (75.0%) were female. A 2-stage random effects approach showed that patients receiving MBCT had a reduced risk of depressive relapse within a 60-week follow-up period compared with those who did not receive MBCT (hazard ratio, 0.69; 95% CI, 0.58-0.82). Furthermore, comparisons with active treatments suggest a reduced risk of depressive relapse within a 60-week follow-up period (hazard ratio, 0.79; 95% CI, 0.64-0.97). Using a 1-stage approach, sociodemographic (ie, age, sex, education, and relationship status) and psychiatric (ie, age at onset and number of previous episodes of depression) variables showed no statistically significant interaction with MBCT treatment. However, there was some evidence to suggest that a greater severity of depressive symptoms prior to treatment was associated with a larger effect of MBCT compared with other treatments.
Conclusions and Relevance
Mindfulness-based cognitive therapy appears efficacious as a treatment for relapse prevention for those with recurrent depression, particularly those with more pronounced residual symptoms. Recommendations are made concerning how future trials can address remaining uncertainties and improve the rigor of the field.
Although progress has been made in the treatment of many psychiatric conditions, recurrent depression continues to cause significant disability and remains a high cost to society.1,2 Interventions that prevent depressive relapse among people at high risk of recurrent episodes have significant potential to reduce the disease’s burden.3 Mindfulness-based cognitive therapy (MBCT), one such intervention, teaches psychological skills that target cognitive mechanisms implicated in depressive relapse to people with a history of depression4 by combining systematic mindfulness training with elements from cognitive therapy. A systematic review and meta-analysis5 of 6 randomized clinical trials (N = 593 patients) suggested that MBCT was associated with a significant reduction in the rates of depressive relapse compared with usual care or placebo, corresponding to a 34% relative risk reduction (risk ratio [RR], 0.66; 95% CI, 0.53-0.82).
While we have a growing body of evidence pointing to the efficacy of MBCT in preventing depressive relapses, we do not know whether MBCT is differentially efficacious for subgroups of people known to be at greater or lesser risk for depressive relapse/recurrence.6,7
Here, we present an analysis of individual patient data (IPD) compiled from 9 published randomized trials of MBCT identified through a systematic literature search. Unlike meta-analyses of aggregate data at the trial level, IPD analyses permit the investigation of patient-level characteristics that may be potential moderators of treatment effects.8 We examined the efficacy of MBCT compared with usual care or active treatment groups for patients from a range of sociodemographic and psychiatric backgrounds participating in studies conducted in a number of countries in Europe and North America, taking into account different periods of follow-up across studies.
Methods
The study was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses statement9 and the good practice guidelines of the Cochrane Collaboration IPD Methods Group10 (eTable 1 in the Supplement).
Study Identification and Data Extraction
We searched for relevant publications from November 2010 (the searching end date of the previous meta-analysis,5 which performed searches from the first available date for each database) to November 2014 (Figure 1) using the same a priori criteria for study inclusion as the previous review, as follows: (1) Study design: randomized trials of MBCT for the prevention of relapse in patients with recurrent major depressive disorder currently in remission, reported in the English language, and published or accepted for publication in peer-reviewed journals; (2) Participants: participants aged 18 years or older, diagnosed as having recurrent major depressive disorder in full or partial remission according to a formal diagnostic classification system (major depressive disorder was defined as a diagnosis based on the DSM-III, -III-R, -IV, or -IV-TR or the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10]); (3) Intervention group: MBCT delivered according to the treatment manual11; (4) Control group: at least 1 non-MBCT treatment, including usual care; and (5) Outcome measures: number of participants meeting the diagnostic criteria for a new major depressive episode over the follow-up study period, according to accepted clinical diagnostic criteria such as the ICD-10 or the DSM-IV-TR.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-analyses Flow Diagram From Record Identification to Study Inclusion.
Studies were identified from searches of titles, abstracts, and keywords of electronic databases (EMBASE, PubMed/Medline, PsycINFO, Web of Science, Scopus, and the Cochrane Controlled Trials Register) using the following search string: (mindfulness-based cognitive therapy) OR (mindfulness based cognitive therapy) OR (MBCT) AND (depress*). No language or other limitations were imposed at this stage. We also checked reference lists of relevant studies and reviews for additional references to potentially relevant studies. This procedure is summarized in Figure 1, and narrative text and an example of a full search string are provided in eTable 2 in the Supplement.
Individual patient data were obtained from the authors of 9 of the 10 trials meeting the inclusion criteria and collated into 1 data set (N = 1329). Overall, IPD integrity was found to be high. The trials are summarized in Table 1, and data extraction and cleaning are elaborated on in eTable 3 in the Supplement. Of the 9 relevant trials, 2 had 3 arms and 7 had 2 arms. One trial included a placebo pill arm18; this small arm (n = 30) was excluded from all analyses. The other 3-arm trial23 had 2 non-MBCT arms: one treatment as usual and the other treatment as usual with cognitive psychological education. For the analyses of MBCT vs non-MBCT, the 2 non-MBCT arms were combined; for the analyses of MBCT vs an active comparator, the treatment as usual arm was excluded. We used the Cochrane risk of bias assessment tool.24 While the risk of bias was generally low across all trials for most criteria (eTable 4 in the Supplement), 2 of 9 trials did not blind assessors17,19 and 1 of these also had incomplete outcome data.17
Table 1. Description of the 9 Primary Studies of MBCT vs Non-MBCT Treatment.
| Source; Study Population and Country | Arm: No. of Participants Randomized | Patients With 60-wk Relapse Status and Time to Relapse, No. (%) | Person-wk Contributed to Unadjusted Analyses | Patients With Final BDI (Closest Available to 60 wk)a | Baseline BDIb, No., Mean (SD) | Mindfulness Measure Used | Patients With Pre- and Posttreatment Mindfulness Scorea | SAEs/SARs |
|---|---|---|---|---|---|---|---|---|
| Teasdale et al,7 2000; Community adults with history of depression, currently in full remission, not receiving ADM at assessment; United Kingdom and Canada |
TAU: 69; MBCT: 76 |
Non-MBCT: 66 (96); MBCT: 70 (92); total % missing: 6 |
Non-MBCT: 2363; MBCT: 3093 |
Non-MBCT: 65; MBCT: 65 |
132, 11.4 (7.9) |
EQ12 | Non-MBCT: 12; MBCT: 14 |
Not formally recorded |
| Ma and Teasdale,6 2004; Community adults with history of depression, currently in full remission, not receiving ADM at assessment; United Kingdom |
TAU: 38; MBCT: 37 |
Non-MBCT: 37 (97); MBCT: 36 (97); total % missing: 3 |
Non-MBCT: 1237; MBCT: 1770 |
Non-MBCT: 33; MBCT: 34 |
73, 13.9 (8.4) |
EQ12 | Non-MBCT: 31; MBCT: 32 |
Not formally recorded |
| Kuyken et al,13 2008; Community adults with history of ≥3 episodes of depression, currently in remission, receiving ADM; United Kingdom |
ADM: 62; MBCT: 61 |
Non-MBCT: 62 (100); MBCT: 61 (100) |
Non-MBCT: 2271; MBCT: 2592 |
Non-MBCT: 58; MBCT: 59 |
123, 19.3 (11.9) |
KIMS14 | Non-MBCT: 58; MBCT: 55 |
No SARs in either arm |
| Bondolfi et al,15 2010; Community adults with history of ≥3episodes of depression, currently in remission, not receiving ADM at assessment; Switzerland |
TAU: 29; MBCT: 31 |
Non-MBCT: 29 (100); MBCT: 31 (100) |
Non-MBCT: 1205; MBCT: 1386 |
Non-MBCT: 26; MBCT: 26 |
60, 9.9 (9.0) |
MAAS16 | Non-MBCT: 29; MBCT: 28 |
Not formally recorded; author communication that none was recorded |
| Godfrin and van Heeringen,17 2010; Community adults with history of ≥3episodes of depression, currently in remission, both receiving and not receiving ADM at assessment; Belgium |
TAU: 54; MBCT: 52 |
Non-MBCT: 47 (87); MBCT: 40 (77); total % missing: 18 |
Non-MBCT: 1690; MBCT: 1964 |
Non-MBCT: 40; MBCT: 35 |
86, 19.9 (12.2) |
MAAS16 | Non-MBCT: 47; MBCT: 37 |
Not formally recorded |
| Segal et al,18 2010c; At point of randomization to MBCT,
community adults with history of depression, currently in full remission
after 8 mo of algorithm-informed ADM in an earlier study
phase; Canada |
Maintenance ADM: 28; MBCT after discontinued ADM: 26 |
Non-MBCT: 28 (100); MBCT: 26 (100) |
Non-MBCT: 1002; MBCT: 1007 |
Non-MBCT: 7; MBCT: 11 |
51, 4.0 (3.9) |
MAAS16 | Non-MBCT: 10; MBCT: 15 |
1 SAE in acute phase (ADM arm) and in the follow-up phase; 0 SAEs in either arm of the trial |
| Huijbers et al,39 2012, and Huijbers et al,19 2015d; Community adults with history of ≥3 episodes of depression, currently in remission, receiving ADM; The Netherlands |
Maintenance ADM: 35; MBCT and ADM: 33 |
Maintenance ADM: 35 (100); MBCT and ADM: 33 (100) |
Non-MBCT: 1342; MBCT: 1433 |
Non-MBCT: 28; MBCT: 28 |
68, 12.1 (9.6) |
FFMQ20 | Non-MBCT: 27; MBCT: 26 |
No SARs in either arm |
| Kuyken et al,21 2015; Community adults with history of ≥3 episodes of depression, currently in remission, receiving ADM; United Kingdom |
Maintenance ADM: 212; MBCT and discontinued ADM: 212 |
Maintenance ADM: 202 (95); MBCT and discontinued ADM: 200 (94); total % missing: 5 |
Non-MBCT: 8882; MBCT: 9471 |
Non-MBCT: 157; MBCT: 167 |
396, 14.1 (10.2) |
FFMQ22 | Non-MBCT: 169; MBCT: 173 |
10 SAEs (5 MBCT arm; 5 in ADM arm), none of which were judged as SAR |
| Williams et al,23 2014; Community adults with history of ≥3 episodes of depression, currently in remission, receiving ADM; United Kingdom |
TAU: 56; CPE: 110; MBCT: 108 |
TAU: 53 (95); CPE: 103 (94); MBCT: 99 (92); total % missing: 7 |
Non-MBCT: 6022; MBCT: 4199 |
Non-MBCT: 135; MBCT: 88 |
255, 8.0 (7.8) |
FFMQ22 | Non-MBCT: 138; MBCT: 87 |
15 SAEs (5 MBCT arm; 10 in CPE arm), of which 1 (CPE) was judged as SAR |
Abbreviations: ADM, antidepressant medication; BDI, Beck Depression Inventory; CPE, cognitive psychological education; EQ, Experiences Questionnaire; FFMQ, Five Facet Mindfulness Questionnaire; KIMS, Kentucky Inventory of Mindfulness Scale; MAAS, Mindful Attention Awareness Scale; MBCT, mindfulness-based cognitive therapy; SAEs, serious adverse events; SARs, serious adverse reactions; TAU, treatment as usual.
Primary outcome data available.
Includes all participants irrespective of trial arm.
Placebo arm excluded.
Huijbers et al19 used Inventory of Depressive Symptomatology (Clinician-Rated).
Primary Outcome
The primary outcome was relapse to depression within 60 weeks of follow-up, collected through a Structured Clinical Diagnostic Interview.25 For studies with a follow-up beyond 60 weeks, follow-up was censored at 60 weeks. From the 9 trials available, participants with data for relapse status and time to relapse measured in weeks were included in all analyses; if relapse did not occur, time to end of follow-up was used. We also reported adverse events.
Sociodemographic and Psychiatric Status Variables
We predefined several sociodemographic characteristics as potential moderators of the effect of MBCT, ie, sex, age, education, relationship status, race/ethnicity, socioeconomic status, and employment status. These variables were standardized across the 9 trials using available data to map each participant to the standardized category (eTable 3 in the Supplement).
Psychiatric status variables included in the moderator analyses were severity of depression symptoms at baseline (measured with the Beck Depression Inventory–II or Inventory of Depressive Symptomatology), baseline mindfulness measured on 1 of several scales, age at onset of depression, and number of previous major depressive episodes.
Statistical Methods
All statistical analyses were conducted according to participants’ randomized allocation in the primary studies. Only complete case data were included for all trials in the main analyses. In the event of substantive missing data (>10%) for an individual trial, a sensitivity analysis was performed using imputed data based on 2 scenarios—one maximally favoring the intervention group and the other maximally favoring the control group—for the 2-stage meta-analysis comparing MBCT with non-MBCT only. All analyses were performed using Stata version 14 (StataCorp LP).
Does MBCT Reduce Depressive Relapse/Recurrence Compared With Control Conditions?
Meta-analyses of time-to-event data were used to evaluate the effect of MBCT compared with non-MBCT on the primary outcome. Both 2-stage and 1-stage meta-analysis methods were used.26 Two-stage methods involved calculating hazard ratios (HRs) for depressive relapse (MBCT vs non-MBCT) for each study individually27,28 and using Cox proportional hazard models, and then combining these HRs in a meta-analysis. Heterogeneity was assessed within 2-stage models using the I2 statistic.29 A 95% CI for the I2 statistic was calculated using the test-based method.30 Both fixed and random effect(s) models were applied.31 Meta-analyses were performed on 3 pairwise comparisons: MBCT vs all non-MBCT treatments, MBCT vs active treatments (antidepressant medication [ADM] or cognitive psychological education), and MBCT vs ADM only.
For 1-stage meta-analyses, both fixed and random effect(s) models were applied to the same 3 pairwise comparisons. Fixed effect models used the Cox proportional hazards model to produce an HR; these models included each individual study as a stratum with its own baseline hazard.32 Where the proportional hazards assumption was unsupported, MBCT status interacting with log(time) was added to the model (and to all subsequent models) to allow the effect of MBCT status on risk of relapse to vary during the follow-up period. Random effects 1-stage models used the Stata command stmixed,33 included a study-level random effect on MBCT status, and applied a flexible parametric survival model.34
Are the Effects of MBCT on Outcomes Moderated by Demographic or Depression-Related Variables?
For our primary outcome of depressive relapse, the use of 1-stage meta-analysis models facilitated inclusion of our sociodemographic and depression-related covariates to investigate moderation.35 The choice of whether to use a fixed effect or random effects approach would be informed by the degree of heterogeneity between studies evident from the 2-stage and 1-stage models comparing MBCT with non-MBCT; in the event of very low heterogeneity, a fixed effect model would be used. A series of multivariable models were created, initially including only the MBCT status of the participant and 1 additional covariate (the interaction between MBCT and log[time] was included if appropriate). As a further check, all covariates were included in 1 overall model to establish which were significantly associated with depressive relapse in the presence of all other covariates. Individual covariates that were found to be statistically significant at the P < .10 threshold in a model including MBCT status only or in a model with all covariates combined were then included in a further model. Covariates that did not achieve significance at the P < .05 level were removed individually from this new model until the most parsimonious model had been ascertained. Each covariate within this model was individually investigated for interaction with MBCT status (ie, each model included only 1 interaction term), and any that were not found to be a significant predictor of time to relapse were individually included in the model with all other significant predictors to investigate potential interaction with MBCT status. In addition, moderation effects between each MBCT status and each individual covariate were investigated in a series of models including only MBCT status, the specified covariate, and their interaction.
Results
Description of Primary Studies
The 9 included studies are described fully in the original trial reports and are summarized here in Table 1. We defined loss to follow-up as a lack of data on relapse status after 60 weeks (or closest available time) of follow-up. Of the 1329 randomized participants from the 9 trials with available IPD, data on relapse status and time to relapse (or end of follow-up with no relapse) were available for 1258 participants (94.7%). Across the sample of 1258 participants, the mean (SD) age was 47.1 (11.9) years (median, 47 years; interquartile range, 39-56), and 944 (75.0%) were female. Of 1230 participants with data available, 509 (41.4%) had at least degree-level qualifications, 636 (51.7%) had qualifications below degree level, and 85 (6.9%) had no qualifications. Of 1239 participants, 726 (58.6%) were married or had a partner, 234 (18.9%) were single, and 279 (22.5%) were divorced, separated, or widowed. Among 1234 participants, the mean (SD) age at onset of depressions was 26.0 (12.2) years (median, 23 years; interquartile range, 17-34), and of 1200 participants, 694 (57.8%) had 5 or more past depressive episodes. Within individual studies, the proportion of participants lost to follow-up ranged from 0% to 18% (Table 1). Of 596 participants who received MBCT, 229 (38%) had a depressive relapse within 60 weeks’ follow-up, whereas 327 of 662 participants (49%) who did not receive MBCT had a depressive relapse within 60 weeks.
Does MBCT Reduce Depressive Relapse Compared With Control Conditions?
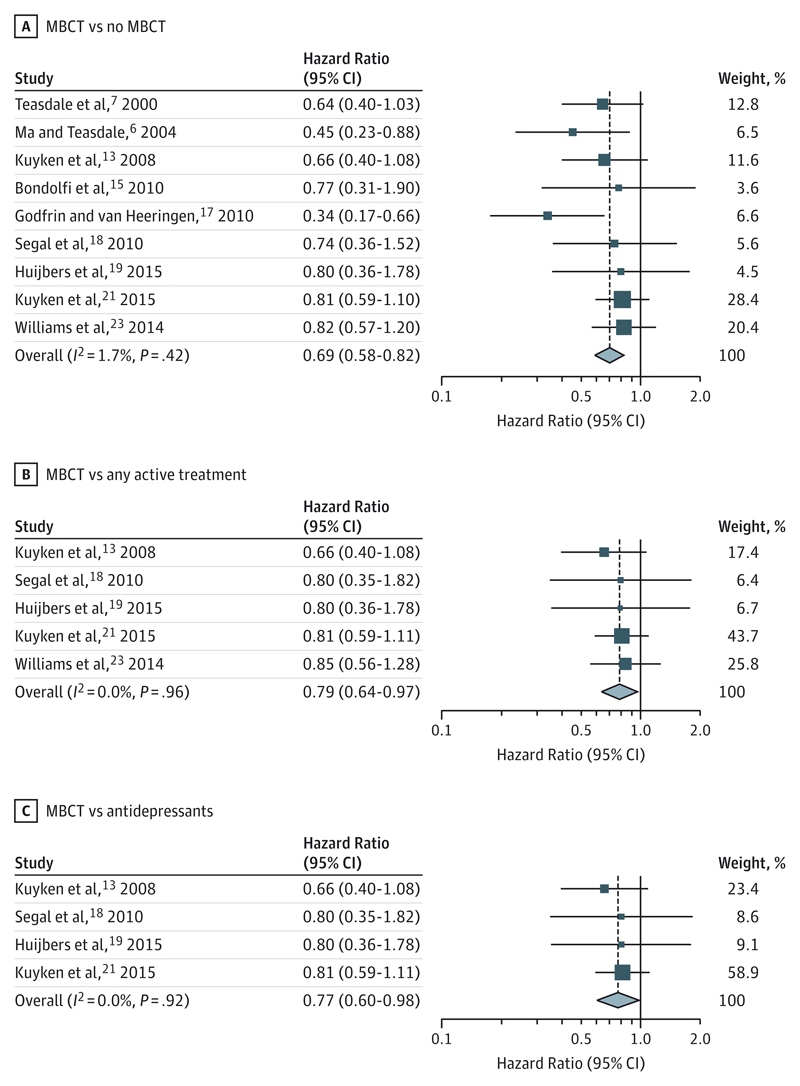
Owing to clinical heterogeneity across the 9 studies, the results of the random effects models are reported; because of very low heterogeneity of treatment effects between studies, the results of equivalent fixed effect analyses were very similar. A forest plot of the 2-stage meta-analysis of time to relapse of depression compared MBCT with all non-MBCT treatments (HR, 0.69; 95% CI, 0.58-0.82; I2, 1.7%; 95% CI, 0-20) (Figure 2A). The funnel plot associated with this analysis indicated some asymmetry, with an absence of smaller studies that showed an increased risk of relapse with MBCT treatment (eFigure 1 in the Supplement). The associated Egger test produced a P value of .18, although we recognize the limited power of this test with only 9 studies. A sensitivity analysis whereby missing outcome data from Godfrin and van Heeringen17 were imputed favoring the MBCT group produced an HR of 0.63 (95% CI, 0.49-0.82); using imputed data that favored the non-MBCT group produced an HR of 0.74 (95% CI, 0.63-0.88). An equivalent analysis comparing MBCT with all active treatments was conducted using data from 5 studies13,18,19,21,23 (HR, 0.79; 95% CI, 0.64-0.97; I2, 0%) (Figure 2B). An analysis comparing MBCT with ADM treatment was conducted using data from 4 studies13,18,19,21 (HR, 0.77; 95% CI, 0.60-0.98; I2, 0%) (Figure 2C). For the latter 2 meta-analyses, the I2 value was 0% in both cases, the lower boundary of the 95% CI was 0%, and the upper boundaries were 43% and 65%, respectively.
Figure 2. Random EffectsMeta-analyses Comparing Mindfulness-Based Cognitive Therapy (MBCT) With Other Variables.
Forest plot of 2-stage meta-analysis of aggregate data on hazard ratio scale comparing (A) risk of relapse of depression in participants receiving MBCT with participants not receiving MBCT; (B) risk of relapse of depression in participants receiving MBCT with participants receiving an alternative active therapy; and (C) risk of relapse of depression in participants receiving MBCT with participants receiving antidepressant medication. Weights are from random effects analyses.
An unadjusted 1-stage fixed effect meta-analysis compared MBCT with non-MBCT treatment (1248 patients, 554 depressive relapses within 60 weeks; HR, 0.69; 95% CI, 0.58-0.82) (Table 2, model A). However, evidence indicated that the proportional hazards assumption was not valid (eFigure 2 in the Supplement shows the log-log plots comparing the MBCT and non-MBCT groups for each of the 9 included studies). Owing to the lack of proportional hazards, the interaction between MBCT status and log(time) was added, allowing the effects of MBCT to vary with log(time). This model (Table 2, model B) yielded an HR for MBCT of 0.34 (95% CI, 0.19-0.60), and for the interaction of MBCT with log(time) of 1.28 (95% CI, 1.06-1.55), this model indicated a reduction in the preventive effect of MBCT on depressive relapse as time progressed during the follow-up period.
Table 2. Cox Proportional Hazards Regression Models and Flexible Parametric Survival Models.
| Covariate | No. | HR (95% CI) | P Value |
|---|---|---|---|
| Model Aa | |||
| Participants | 1248 | NA | NA |
| Depressive relapses | 554 | NA | NA |
| MBCT status (reference: non-MBCT) | NA | 0.69 (0.58-0.82) | <.001 |
| Model Ba | |||
| Participants | 1248 | NA | NA |
| Depressive relapses | 554 | NA | NA |
| MBCT status (reference: non-MBCT) | NA | 0.34 (0.19-0.60) | <.001 |
| MBCT by log(time)b | NA | 1.28 (1.06-1.55) | .01 |
| Model Ca | |||
| Participants | 892 | NA | NA |
| Depressive relapses | 385 | NA | NA |
| MBCT status (reference: active treatments) | NA | 0.78 (0.64-0.96) | .02 |
| Model Da | |||
| Participants | 637 | NA | NA |
| Depressive relapses | 266 | NA | NA |
| MBCT status (reference: antidepressant medication) | NA | 0.77 (0.60-0.98) | .03 |
| Model Ec | |||
| Participants | 1248 | NA | NA |
| Depressive relapses | 554 | NA | NA |
| Between-study SD | 0.0008 | NA | NA |
| MBCT status (reference: non-MBCT) | NA | 0.68 (0.58-0.81) | <.001 |
| Model Fd | |||
| Participants | 1248 | NA | NA |
| Depressive relapses | 554 | NA | NA |
| Between-study SD | 0.0007 | NA | NA |
| MBCT status (reference: non-MBCT) | NA | 0.63 (0.53-0.76) | <.001 |
| Model Ga,e | |||
| Participants | 1022 | NA | NA |
| Depressive relapses | 443 | NA | NA |
| MBCT status (reference: non-MBCT) | NA | 0.74 (0.61-0.90) | .003 |
| Baseline depression score | NA | 1.40 (1.24-1.58) | <.001 |
| Baseline depression score by MBCT status | NA | 0.80 (0.66-0.97) | .02 |
Abbreviations: HR, hazard ratio; MBCT, mindfulness-based cognitive therapy; NA, not applicable.
Cox proportional hazards regression model stratified by individual study.
Time measured in weeks.
Flexible parametric model with 2 df and random treatment effects.
Based on model E, with the inclusion of interaction between MBCT status and restricted cubic splines to account for the time-varying effect of MBCT (P = .04).
Model adjusted for baseline mindfulness z score, age at onset of depression, and number of past episodes of depression (5 or more/4 or fewer).
A 1-stage fixed effect model using 5 studies13,18,19,21,23 compared MBCT with active treatments only (892 participants and 385 relapses; HR, 0.78; 95% CI, 0.64-0.96) (Table 2, model C) and was very similar to the 2-stage random effects model, which provided little evidence to indicate lack of proportional hazards. The equivalent analysis comparing MBCT with ADM treatment used 4 studies13,18,19,21 (637 participants and 266 relapses; HR, 0.77; 95% CI, 0.60-0.98) (Table 2, model D) and was identical to the results of the 2-stage random effects model, also with little evidence to support lack of proportional hazards.
The 1-stage random effects model compared MBCT with all non-MBCT treatments using a flexible parametric model with 2 df (HR, 0.68; 95% CI, 0.58-0.81; between-study SD, 0.0008) (Table 2, model E). A further model comparing MBCT with non-MBCT was created by adding the interaction between MBCT status and the restricted cubic splines derived from the previous model (HR, 0.63; 95% CI, 0.53-0.76; between-study SD, 0.0007) (Table 2, model F); the global P value for the interaction between MBCT status and each restricted cubic spline was .04, consistent with a significant time-varying effect of MBCT status observed in the fixed effect model.
Equivalent analyses comparing MBCT with all active treatments and comparing MBCT with ADM, with or without a time-varying effect on MBCT status, failed to converge, almost certainly owing to very low heterogeneity between studies.
Are the Effects of MBCT on Outcomes Moderated by Demographic and Depression-Related Variables?
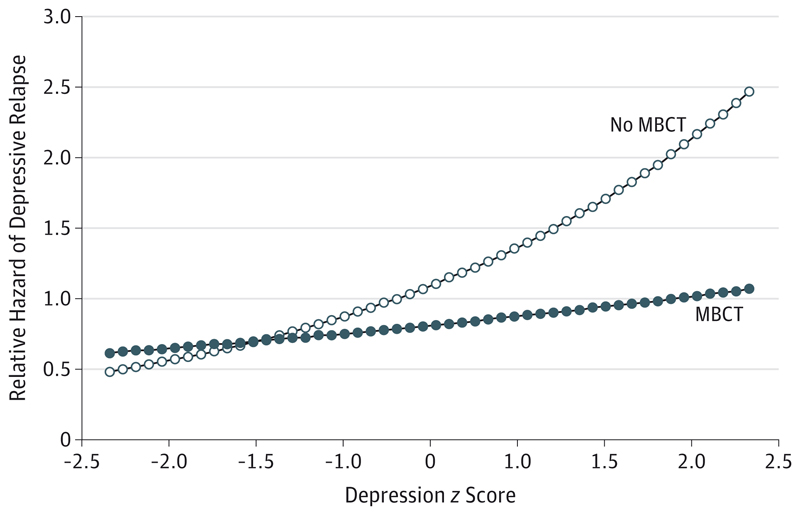
In view of the low heterogeneity between studies, fixed effect 1-stage models were used for the moderation analyses. Individually, 5 sociodemographic and psychiatric variables were found to be significantly associated with risk of relapse within 60 weeks: baseline depression z score, baseline mindfulness z score, age at onset, number of previous episodes, and marital status (all P < .10). With the exception of marital status, all of these covariates were also significantly associated with time to relapse when included in a model with MBCT status and its interaction with log(time) and all other covariates. When included in a model with MBCT status and its interaction with log(time), only 4 remained statistically significant: baseline depression z score, baseline mindfulness z score, age at onset, and number of previous episodes. However, on including these 4 covariates, the interaction between MBCT status and log(time) was no longer significant (P = .052), so it was removed from the model. Thus, the significant predictors of depressive relapse were baseline depression z score, baseline mindfulness z score, age at onset, and number of previous episodes. When including the interaction with MBCT and each predictor in turn into this model, baseline depression z score had a significant interaction with MBCT status (Table 2, model G; Figure 3); patients with a higher baseline depression z score received greater benefit from MBCT therapy compared with all non-MBCT treatments. Of the remaining significant covariates, only baseline mindfulness z score had a significant interaction with MBCT status both in a model with no other covariates and in a model with all other significant covariates. However, these interactions became nonsignificant when the interaction between MBCT status and baseline depression z score was added to the model. No other covariates were found to have a significant interaction with MBCT status when included in a model with all other significant covariates or in a model with only the respective covariate, MBCT status, their interaction, and the interaction between MBCT status and log(time).
Figure 3. Interactive Effect Between Mindfulness-Based Cognitive Therapy (MBCT) Status and Baseline Depression With Regard to the Relative Hazard of Depressive Relapse.
Predictive margins for the relative hazard of depressive relapse comparing participants receiving MBCT with those not receiving MBCT at baseline depression z scores, derived from a model including MBCT status, baseline depression z score, the interaction between MBCT status and baseline depression z score, baseline mindfulness z score, age at onset of depression, and number of past episodes of depression (5 or more/4 or fewer).
Discussion
Summary of Results
Replicating previous work, we found clear evidence that MBCT was associated with a significant reduction in the risk of depressive relapse/recurrence over 60 weeks compared with usual care. Extending previous work, we found that MBCT reduces the risk of depressive relapse/recurrence compared with the current mainstay approach, maintenance antidepressants. We further showed that there is no support for MBCT having differential effects for patients based on their sex, age, education, or relationship status, suggesting the intervention’s generalizability across these characteristics. Different research groups conducted the 9 randomized clinical trials and used different clinicians across a range of European and North American countries. The lack of heterogeneity between studies in effects on time to depressive relapse suggests that the effects of MBCT are similar in these different contexts.
Mindfulness-based cognitive therapy was developed for patients in remission but at high risk for depressive relapse/recurrence. Our analyses suggest that the treatment effect of MBCT on the risk of depressive relapse/recurrence is larger in participants with higher levels of depression symptoms at baseline compared with non-MBCT treatments, suggesting that MBCT may be particularly helpful to those who still have significant depressive symptoms. This is consistent with several recent trials that suggest MBCT may be more effective for people whose depressive symptoms fluctuate18 and/or who report a history of early adversity.21,23 Adverse events were formally recorded in 6 of 9 studies, but none were attributed to MBCT.
Strengths and Limitations of the Study
To address the question of whether treatment effects are influenced by individual patient characteristics, a study needs to be adequately powered and use appropriate statistical approaches. Within the constraints of the constituent studies, our IPD approach provided an opportunity to answer these questions. Risk of bias was low, suggesting confidence in these findings. Combining a series of modest-sized trials with effects in the predicted direction but missing significance individually yields a significant combined estimate of effect.
We did observe asymmetry in the funnel plot with an absence of smaller studies that showed an increased risk of relapse with MBCT treatment. It is possible that there are unpublished studies that we are not aware of, and we welcome investigators of any such studies to bring them to our attention so that their data can be included in future updates. The unavailability of the Meadows et al36 study data represents an impediment to IPD, transparency, and external scrutiny. Funding bodies, ethics committees, and sponsors should work to a consensus position. Finally, allegiance effects can influence effect sizes in psychological therapy trials,37 and the constituent trials were largely conducted by proponents of MBCT. Therefore, we analyzed risk of bias (eTable 4 in the Supplement).
There were a number of limitations resulting from availability of data within the constituent studies. For example, we were not able to obtain information about important potential moderators such as race/ethnicity and employment. Trials also vary in the way data are collected. For example, age at first onset of depression was collected in some trials by simple self-report and in others through standardized Structured Clinical Interview. Number of prior episodes was also gathered inconsistently. Adverse events were not systematically recorded or reported. As with all meta-analyses, there may be trials published in other languages or unpublished trials we were not able to access. Moderator analyses were not formally powered, exploratory, or relatively large in number, increasing the risk of type I errors. Future studies should plan and power for moderator analyses.
Conclusions
While previous research has shown the superiority of MBCT compared with usual care,5 this study provides important new evidence that MBCT is also effective compared with other active treatments and that its effects are not restricted to particular groups defined by age, educational level, marital status, or sex. A recent meta-analysis38 of the effectiveness of all psychological interventions to prevent recurrence compared with usual care and antidepressants suggests that the protective effects of MBCT are comparable with those for cognitive therapy (vs usual care: RR, 0.68; 95% CI, 0.54-0.87; vs ADM: RR, 0.08; 95% CI, 0.61-1.02) and interpersonal therapy (vs usual care: RR, 0.41; 95% CI, 0.27-0.63; vs ADM: RR, 0.08; 95% CI, 0.50-1.38). However, MBCT addresses a particular clinical problem, namely teaching skills to stay well to people currently well but at high risk of depressive relapse. There is a reduction in protective effects over time. The finding that MBCT may be most helpful for patients with higher levels of depressive symptoms adds to an emerging consensus that the greater the risk for depressive relapse/recurrence, the more benefit MBCT offers. Patients with lower baseline scores appeared to receive less benefit but were not disadvantaged by MBCT.
We recommend that future trials consider an active control group, use comparable primary and secondary outcomes (Structured Clinical Interview for DSM for depressive relapse), use longer follow-ups, report treatment fidelity, collect key background variables (eg, race/ethnicity and employment), take care to ensure generalizability, conduct cost-effectiveness analyses, put in place ethical and data man-agement procedures that enable data sharing, consider mechanisms of action, and systematically record and report adverse events.
Supplementary Material
Key Points.
Question
What is the efficacy of mindfulness-based cognitive therapy compared with usual care and other treatments?
Findings
This individual patient data meta-analysis included 9 trials, comprising 1329 participants. Patients receiving mindfulness-based cognitive therapy had a significantly reduced risk of depressive relapse within a 60-week follow-up period compared with those who received usual care and had comparable outcomes to those who received other active treatments.
Meaning
Mindfulness-based cognitive therapy appears efficacious as a treatment for relapse prevention for those who have recurrent depression and provides an alternative choice to other active treatments.
Funding/Support
This work was supported by Wellcome Trust grants GR067797 and 104908/Z/14/Z. Drs Kuyken, Taylor, Byford, and Byng were partially supported by the National Institute for Health Research Healthy Technology Assessment program. Drs Taylor and Byng have also been supported by the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care South West Peninsula at the Royal Devon and Exeter National Health Service Foundation Trust. Drs Schweizer and Dalgleish were supported by the Medical Research Council.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Kuyken had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kuyken, Warren, Taylor, Whalley, Dalgleish.
Acquisition, analysis, or interpretation of data: Dalgleish, Schweizer, Warren.
Drafting of the manuscript: Kuyken, Warren, Taylor, Whalley, Crane, Schweizer, Dalgleish.
Critical revision of the manuscript for important intellectual content: All authors.
Study supervision: Taylor.
Conflict of Interest Disclosures: All authors with the exception of Drs Warren (independent statistician) and Schweizer (independent systematic reviewer) were investigators on 1 or more of the original randomized clinical trials that contributed data to the individual patient data and secured grant funding for these trials. Dr Williams founded the Oxford Mindfulness Centre and was its director until 2013. Dr Kuyken is its current director. Dr Speckens is founder and clinical director of the Radboud UMC Centre for Mindfulness and Dr Ma is director of the Centre for Mindfulness, Hong Kong. Dr Crane and Ms Huijbers are affiliated with the Oxford and Radboud University–based mindfulness centers, respectively. Drs Teasdale, Williams, and Segal receive royalties for books on mindfulness-based cognitive therapy that they have coauthored. Drs Williams, Kuyken, Speckens, Ma, and Segal additionally receive payments for training workshops and presentations related to mindfulness-based cognitive therapy. Dr Kuyken donates all such fees to the Oxford Mindfulness Foundation, a charitable trust that supports the work of the Oxford Mindfulness Centre, as does Dr Speckens to the Radboud UMC. Dr Segal is a member of the scientific advisory board for Mindful Noggin, which is part of NogginLabs, a private company specializing in customized web-based learning. Dr Kuyken was an unpaid director of the Mindfulness Network Community Interest Company until 2015. Drs Byng, Kuyken, and Williams gave evidence to the UK Mindfulness All Party Parliamentary Group. No other disclosures were reported.
Disclaimer: The views and opinions expressed in this article are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the UK Department of Health.
Additional Contributions: We are grateful to the trial teams of the constituent trials in this individual patient data analysis and to Daniel Brett, MSc (Department of Psychiatry, University of Oxford, Prince of Wales International Centre, Warneford Hospital, Oxford, England), for administrative assistance. Mr Brett did not receive additional compensation for his contributions.
Contributor Information
Willem Kuyken, Department of Psychiatry, University of Oxford, Prince of Wales International Centre, Warneford Hospital, Oxford, England.
Ben Whalley, School of Psychology, Faculty of Health and Human Sciences, University of Plymouth, Plymouth, England.
Catherine Crane, Department of Psychiatry, University of Oxford, Prince of Wales International Centre, Warneford Hospital, Oxford, England; Department of Psychiatry, University of Oxford, Prince of Wales International Centre,Warneford Hospital, Oxford, England.
Guido Bondolfi, Department of Psychiatry, University Medical Centre, University of Geneva, Geneva, Switzerland.
Rachel Hayes, Institute of Health Research, Child Health Group, Exeter Medical School, Exeter, England.
Marloes Huijbers, Department of Psychiatry, Radboud University Nijmegen Medical Centre, Radboud University Nijmegen, Nijmegen, The Netherlands.
Helen Ma, Department of Psychiatry, University of Oxford, Prince of Wales International Centre, Warneford Hospital, Oxford, England; Hong Kong Centre for Mindfulness, Hong Kong.
Susanne Schweizer, Medical Research Council Cognition and Brain Sciences Unit, Cambridge, England.
Zindel Segal, Department of Psychology, University of Toronto Scarborough, Toronto, Ontario, Canada.
Anne Speckens, Department of Psychiatry, Radboud University Nijmegen Medical Centre, Radboud University Nijmegen, Nijmegen, The Netherlands.
John D. Teasdale, Medical Research Council Cognition and Brain Sciences Unit, Cambridge, England.
Kees Van Heeringen, University Department of Psychiatry, University Hospital, Gent, Belgium.
Mark Williams, Department of Psychiatry, University of Oxford, Prince of Wales International Centre, Warneford Hospital, Oxford, England.
Sarah Byford, King’s Health Economics, King’s College London, London, England.
Richard Byng, Peninsula School of Medicine, Plymouth University, Plymouth, England.
Tim Dalgleish, Hong Kong Centre for Mindfulness, Hong Kong; Medical Research Council Cognition and Brain Sciences Unit, Cambridge, England; Cambridgeshire and Peterborough NHS Foundation Trust, Cambridge, England.
References
- 1.Collins PY, Patel V, Joestl SS, et al. Scientific Advisory Board and the Executive Committee of the Grand Challenges on Global Mental Health. Grand challenges in global mental health. Nature. 2011;475(7354):27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Layard R. The case for psychological treatment centres. BMJ. 2006;332(7548):1030–1032. doi: 10.1136/bmj.332.7548.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuijpers P, van Straten A, Smit F, Mihalopoulos C, Beekman A. Preventing the onset of depressive disorders: a meta-analytic review of psychological interventions. Am J Psychiatry. 2008;165(10):1272–1280. doi: 10.1176/appi.ajp.2008.07091422. [DOI] [PubMed] [Google Scholar]
- 4.Segal ZV, Williams JMG, Teasdale JD. Mindfulness-Based Cognitive Therapy for Depression: A New Approach to Preventing Relapse. New York, NY: Guilford Press; 2002. [Google Scholar]
- 5.Piet J, Hougaard E. The effect of mindfulness-based cognitive therapy for prevention of relapse in recurrent major depressive disorder: a systematic review and meta-analysis. Clin Psychol Rev. 2011;31(6):1032–1040. doi: 10.1016/j.cpr.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Ma SH, Teasdale JD. Mindfulness-based cognitive therapy for depression: replication and exploration of differential relapse prevention effects. J Consult Clin Psychol. 2004;72(1):31–40. doi: 10.1037/0022-006X.72.1.31. [DOI] [PubMed] [Google Scholar]
- 7.Teasdale JD, Segal ZV, Williams JMG, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol. 2000;68(4):615–623. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- 8.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2010;340 doi: 10.1136/bmj.c221. c221. [DOI] [PubMed] [Google Scholar]
- 9.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 10.Stewart LA, Clarke M, Rovers M, et al. PRISMA-IPD Development Group. Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. JAMA. 2015;313(16):1657–1665. doi: 10.1001/jama.2015.3656. [DOI] [PubMed] [Google Scholar]
- 11.Segal ZV, Williams JMG, Teasdale JD. Mindfulness-Based Cognitive Therapy for Depression. 2nd ed. New York, NY: Guilford Press; 2013. [Google Scholar]
- 12.Fresco DM, Moore MT, van Dulmen MHM, et al. Initial psychometric properties of the Experiences Questionnaire: validation of a self-report measure of decentering. Behav Ther. 2007;38(3):234–246. doi: 10.1016/j.beth.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Kuyken W, Byford S, Taylor RS, et al. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. J Consult Clin Psychol. 2008;76(6):966–978. doi: 10.1037/a0013786. [DOI] [PubMed] [Google Scholar]
- 14.Baer RA, Smith GT, Allen KB. Assessment of mindfulness by self-report: the Kentucky Inventory of Mindfulness Skills. Assessment. 2004;11(3):191–206. doi: 10.1177/1073191104268029. [DOI] [PubMed] [Google Scholar]
- 15.Bondolfi G, Jermann F, der Linden MV, et al. Depression relapse prophylaxis with mindfulness-based cognitive therapy: replication and extension in the Swiss health care system. J Affect Disord. 2010;122(3):224–231. doi: 10.1016/j.jad.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. J Pers Soc Psychol. 2003;84(4):822–848. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- 17.Godfrin KA, van Heeringen C. The effects of mindfulness-based cognitive therapy on recurrence of depressive episodes, mental health and quality of life: a randomized controlled study. Behav Res Ther. 2010;48(8):738–746. doi: 10.1016/j.brat.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Segal ZV, Bieling P, Young T, et al. Antidepressant monotherapy vs sequential pharmacotherapy and mindfulness-based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression. Arch Gen Psychiatry. 2010;67(12):1256–1264. doi: 10.1001/archgenpsychiatry.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huijbers MJ, Spinhoven P, Spijker J, et al. Adding mindfulness-based cognitive therapy to maintenance antidepressant medication for prevention of relapse/recurrence in major depressive disorder: randomised controlled trial. J Affect Disord. 2015;187:54–61. doi: 10.1016/j.jad.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 20.Veehof MM, Ten Klooster PM, Taal E, Westerhof GJ, Bohlmeijer ET. Psychometric properties of the Dutch Five Facet Mindfulness Questionnaire (FFMQ) in patients with fibromyalgia. Clin Rheumatol. 2011;30(8):1045–1054. doi: 10.1007/s10067-011-1690-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuyken W, Hayes R, Barrett B, et al. Effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse or recurrence (PREVENT): a randomised controlled trial. Lancet. 2015;386(9988):63–73. doi: 10.1016/S0140-6736(14)62222-4. [DOI] [PubMed] [Google Scholar]
- 22.Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13(1):27–45. doi: 10.1177/1073191105283504. [DOI] [PubMed] [Google Scholar]
- 23.Williams JM, Crane C, Barnhofer T, et al. Mindfulness-based cognitive therapy for preventing relapse in recurrent depression: a randomized dismantling trial. J Consult Clin Psychol. 2014;82(2):275–286. doi: 10.1037/a0035036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.First MB, Spitzer RL, Gibbon M, Williams JBW. The Structured Clinical Interview for DSM-IV Axis I Disorder With Psychotic Screen. New York, NY: New York Psychiatric Institute; 1995. [Google Scholar]
- 26.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-analysis. Chichester, England: John Wiley & Sons, Ltd; 2009. [Google Scholar]
- 27.Tudur C, Williamson PR, Khan S, Best LY. The value of the aggregate data approach in meta-analysis with time-to-event outcomes. J Roy Stat Soc A Sta. 2001;164(2):357–370. doi: 10.1111/1467-985X.00207. [DOI] [Google Scholar]
- 28.Williamson PR, Smith CT, Hutton JL, Marson AG. Aggregate data meta-analysis with time-to-event outcomes. Stat Med. 2002;21(22):3337–3351. doi: 10.1002/sim.1303. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 31.Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342 doi: 10.1136/bmj.d549. d549. [DOI] [PubMed] [Google Scholar]
- 32.Smith CT, Williamson PR, Marson AG. Investigating heterogeneity in an individual patient data meta-analysis of time to event outcomes. Stat Med. 2005;24(9):1307–1319. doi: 10.1002/sim.2050. [DOI] [PubMed] [Google Scholar]
- 33.Crowther MJ, Look MP, Riley RD. Multilevel mixed effects parametric survival models using adaptive Gauss-Hermite quadrature with application to recurrent events and individual participant data meta-analysis. Stat Med. 2014;33(22):3844–3858. doi: 10.1002/sim.6191. [DOI] [PubMed] [Google Scholar]
- 34.Royston P, Parmar MKB. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21(15):2175–2197. doi: 10.1002/sim.1203. [DOI] [PubMed] [Google Scholar]
- 35.Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59(10):877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- 36.Meadows GN, Shawyer F, Enticott JC, et al. Mindfulness-based cognitive therapy for recurrent depression: a translational research study with 2-year follow-up. Aust N Z J Psychiatry. 2014;48(8):743–755. doi: 10.1177/0004867414525841. [DOI] [PubMed] [Google Scholar]
- 37.Driessen E, Hollon SD, Bockting CLH, Cuijpers P, Turner EH. Does publication bias inflate the apparent efficacy of psychological treatment for major depressive disorder? a systematic review and meta-analysis of US National Institutes of Health-funded trials. PLoS One. 2015;10(9):e0137864. doi: 10.1371/journal.pone.0137864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biesheuvel-Leliefeld KE, Kok GD, Bockting CL, et al. Effectiveness of psychological interventions in preventing recurrence of depressive disorder: meta-analysis and meta-regression. J Affect Disord. 2015;174:400–410. doi: 10.1016/j.jad.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 39.Huijbers MJ, Spijker J, Donders AR, et al. Preventing relapse in recurrent depression using mindfulness-based cognitive therapy, antidepressant medication or the combination: trial design and protocol of the MOMENT Study. BMC Psychiatry. 2012;12:125. doi: 10.1186/1471-244X-12-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.