Abstract
Assaying for enzymatic activity is a persistent bottleneck in biocatalyst and drug development. Existing high-throughput assays for enzyme activity tend to be applicable only to a narrow range of biochemical transformations, whereas universal enzyme characterization methods usually require chromatography to determine substrate turnover, greatly diminishing throughput. We present an enzyme activity assay which allows for the high-throughput mass-spectrometric detection of enzyme activity in complex matrices without the need for a chromatographic step. We demonstrate that this technology, which we call “Probing Enzymes with ‘Click’–Assisted NIMS” (PECAN), can detect the activity of medically and biocatalytically significant cytochrome P450s in cell lysate, microsomes, and bacterial cells. Using our approach, we successfully screened a cytochrome P450BM3 mutant library for the ability to catalyze the oxidation of the sesquiterpene valencene.
Keywords: High-throughput screening, Mass spectrometry, Enzyme assays, Biocatalysis, Cytochrome P450
Graphical Abstract
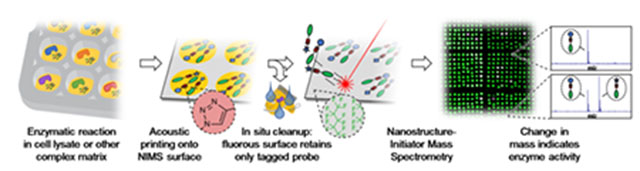
Easy as pecan pie: A high-throughput enzyme assay was developed, based on a combination of click chemistry, fluorous affinity purification, and Nanostructure-Initiator Mass Spectrometry. The utility of the technique, called PECAN, was demonstrated by screening a library of cytochrome P450s for activity on valencene.
Enzyme screening campaigns are frequently the bottleneck of drug, biomarker and biocatalyst discovery programs, making high-throughput enzyme activity assays essential to accelerating research in these areas[1–4]. Such screens often rely on fluorogenic or chromogenic enzyme substrate analogs, coupled assays, or biosensors, which – while high-throughput – are unsuitable for monitoring many important enzymatic transformations[5–7].
Mass spectrometry (MS) is a promising technology for the analysis of enzyme activity, given that the majority of enzymatic reactions change their substrates’ masses. However, due to ionization suppression and isobaric ions, MS analysis is limited to low-complexity samples. This poses a significant disadvantage when screening large enzyme libraries for a desired catalytic activity, or evaluating enzymatic activity exhibited by clinical samples, both of which are typically conducted in complex biological matrices. Coupling MS analysis to a chromatographic step, such as in liquid chromatography-MS (LC-MS) or gas chromatography-MS (GC-MS), alleviates ionization and spectral complexity issues by physically separating analytes, but significantly decreases throughput.
One promising platform for high-throughput enzyme activity determination is Nanostructure-Initiator Mass Spectrometry (NIMS)[8–10]. NIMS involves laser desorption of analytes from a fluorophilic surface. Fortuitously, this surface has high affinity for perfluoroalkylated analytes through non-covalent fluorous interactions, enabling the substitution of lengthy chromatographic separations with an in-situ washing step. However, many enzymes may be unable to accommodate perfluoroalkylated substrates in their active sites, which may be why NIMS-based enzyme assays have thus far been applied only to carbohydrate-acting enzymes and acetyltransferases[8,9,11–13].
In this work, we carry out an enzymatic reaction on a substrate analog (“probe”), after which we couple the probe to a perfluoroalkylated affinity tag using copper-catalyzed “click” chemistry[14] (Fig. 1). This approach, which we call “Probing Enzymes with ‘Click’–Assisted NIMS” (PECAN), requires only the incorporation of a relatively small “clickable” handle into the probe. When combined with acoustic sample deposition and MS Imaging[15], this permits high–throughput characterization of enzyme activity in complex biological matrices.
Figure 1.
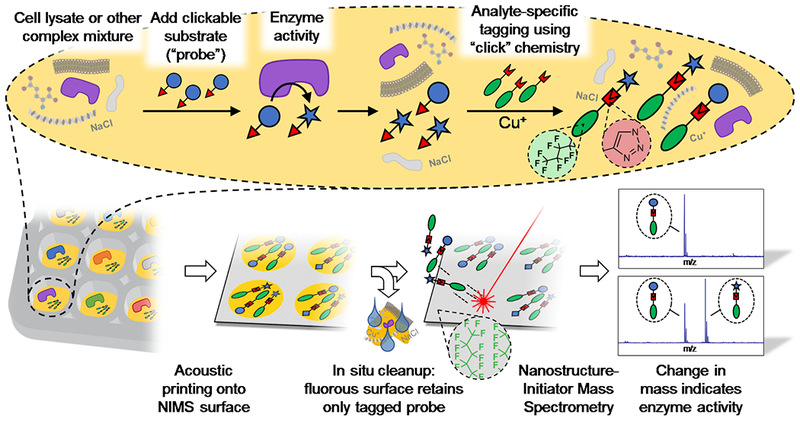
Outline of the PECAN technology. The Nanostructure-Initiator Mass Spectrometry (NIMS) surface allows for high-throughput enzyme activity determination in complex samples, circumventing chromatographic separations by means of in situ fluorous affinity purification of perfluorinated analytes. In PECAN, a perfluorinated tag is covalently attached to the enzyme substrate and product(s) using Cu(I)-catalyzed “click” chemistry, after completion of the enzyme reaction. Following the cleanup step, retained analytes are analyzed directly by NIMS.
We apply PECAN to cytochrome P450s (“P450s”) which are an important class of enzymes possessing the remarkable capacity to effect regio– and stereospecific C–H bond activation reactions. P450s play important roles in the biosynthesis of natural products[16] and steroids[17], and are central to human xenobiotic metabolism[18]. Their ability to catalyze reactions that are outside of the reach of traditional synthetic organic chemistry has made P450s valuable biocatalysts. Consequently, much research has been directed towards identifying P450s with the appropriate substrate–, regio– and stereospecificity for industrial biotransformations[19–21]. High-throughput P450 activity assays developed to facilitate these studies include chromogenic[22–24], fluorometric[25], luminogenic[26] and coupled assays[27], as well as MS-based approaches harnessing the high mass resolution of FT-ICR MS to distinguish analytes from matrix ions[28] or solid-phase extraction to enrich for analytes prior to MS analysis[29].
To benchmark the PECAN technology, we tested its ability to detect the activity of cytochrome P450BM3, a model P450 widely employed in biocatalysis[30]. P450BM3 is known to catalyze the hydroxylation of 1a to form 2a with a 34% e.e. of R-2a[31] (Fig. 2a), We designed an analog of 1a harboring an azide, 1b, to act as PECAN probe (Fig. 2b). We fed 1b (0.5 mM in 1% v/v DMSO) to lysates of E. coli expressing P450BM3 or Green Fluorescent Protein (GFP, serving as a negative control), along with a cofactor regeneration system (10 mM glucose 6-phosphate, 100 µM NADP+ and 0.1 unit/mL G6P dehydrogenase). After a 3-hour enzymatic reaction, the lysates were tagged with a perfluorinated alkyne through a copper(I)-catalyzed “click” reaction, and acoustically transferred onto a NIMS surface. The surface was washed with water and rastered on a MALDI-TOF mass spectrometer. The resulting MS image showed excellent signal-to-noise (Fig. 2c), and P450BM3 reactions could easily be distinguished from negative controls both qualitatively and quantitatively (Fig. 2d). The Z-factor – a commonly-used statistical measure of the quality a high-throughput assay[32] – was calculated to be 0.93, indicating an excellent assay. Similar results were obtained for biological replicates assayed in 96-deepwell microtiter plate format (Fig. S1), suggesting that PECAN can be applied to high-throughput enzyme screening experiments.
Figure 2.
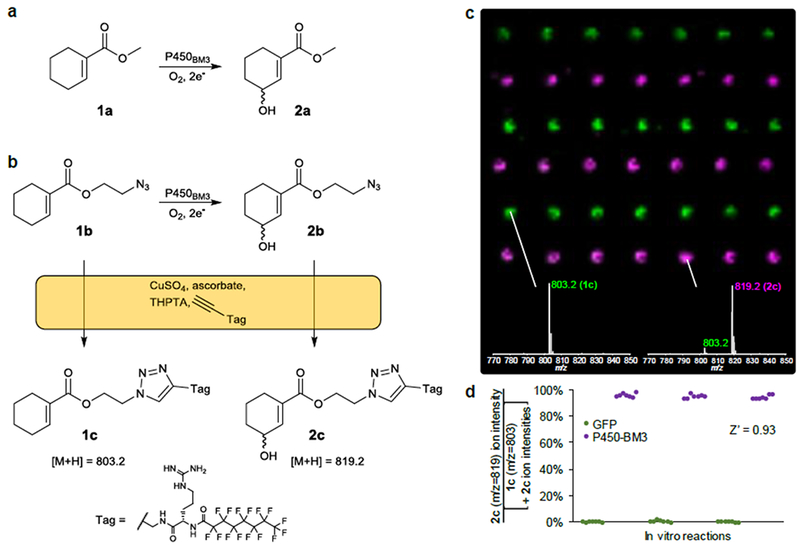
Benchmarking of the PECAN technology. a) Wild-type cytochrome P450BM3 is known to catalyze the hydroxylation of 1a. b) An analog of 1a, 1b, was used as the substrate probe for the PECAN experiment. Upon tagging 1b and its hydroxylation product 2b using Cu(I)-catalyzed “click” chemistry, 1c and 2c are formed, respectively. c) MS image showing the spatial distribution of ions with m/z = 803 (green, 1c) and m/z = 819 (violet, 2c), generated using OpenMSI[39]. Each of the pixels’ two color intensities maps linearly to their respective ion’s percentile intensity in the MS image. Lysates of 3 E. coli cultures expressing GFP (negative controls) and 3 cultures expressing P450BM3 (rows, alternating) were left to act on 1b for 3h. Each lysate underwent a tagging reaction, and 1 nL of each reaction was printed onto a NIMS surface 7 times (columns). Inset mass spectra are single-pixel spectra representative of the spot. Distance between spots (pitch) is 0.75 mm. d) Percent turnover (assuming 1c and 2c ionize similarly) was calculated from the spots’ 803 and 819 ion intensities (area under the MS curve). Data points (representing individual spots) are grouped by enzymatic reaction (i.e., rows in c).
We also investigated whether PECAN could be used to monitor intracellular enzymatic reactions. Probing enzymatic activity in vivo rather than in lysate increases the relevance of screens to downstream whole-cell bioconversion applications, could improve experimental throughput by avoiding the lysis procedure, and decreases assay costs by avoiding the need for exogenous cofactors. Using PECAN, we could successfully monitor the oxidation of 1b fed to whole E. coli cells expressing P450BM3 (Fig. S2).
Eukaryotic P450s are typically membrane-bound and studied in microsomes (membrane fractions derived from the endoplasmic reticulum), prompting us to test the applicability of PECAN to the measurement of P450 activity in microsomes. We subjected the contraceptive medication 19-norethindrone to recombinant human microsomal P450 CYP3A4 and were able to detect its oxidation products (Fig. S3). Unlike 1b, which contains an azide, 19-norethindrone harbors an alkyne, requiring a reversal of the polarity of the tagging “click” reaction. This did not noticeably affect the efficiency of the tagging reaction or MS signal. CYP3A4 activity could be abolished in the presence of the known inhibitor clotrimazole (Fig. S3), suggesting that that PECAN may be suitable for drug-drug interaction screening.
As a model high-throughput enzyme screening campaign, we aimed to identify mutants of P450BM3 with the ability to oxidize valencene (3a) to the fragrance and insect repellent nootkatone (5a)[33], a commonly-studied biocatalytic process[34]. In such a process, a P450 hydroxylates 3a, producing either epimer of 4a. The P450 then again oxidizes the same position on 4a to yield 5a (Fig. 3a). While wild-type P450BM3 is unable to effect this transformation, mutants displaying this ability have been discovered in studies evaluating small, focused mutant libraries[35,36]. Although several P450BM3 variants were found to act on 3a, most are either unable to oxidize it beyond 4a, or oxidize 3a at more than one position, yielding non-specifically over-oxidized products (Figs. 3a, S4). Although PECAN cannot distinguish between isomers, it can estimate the extent of probe oxidation from its mass distribution, and thereby help reveal the library’s most promising members. While peak heights of different ions in a mass spectrum cannot be directly quantified in the absence of isotopically-labeled internal standards, we expect that the analytes’ desorption and ionization in the PECAN assay is largely determined by the tag, which is shared by all analytes. Regardless, the MS signal of an ion proportional to all other tagged ions is expected to rise monotonically with its concentration, allowing us to identify the most productive wells in a screen based on ion counts.
Figure 3.
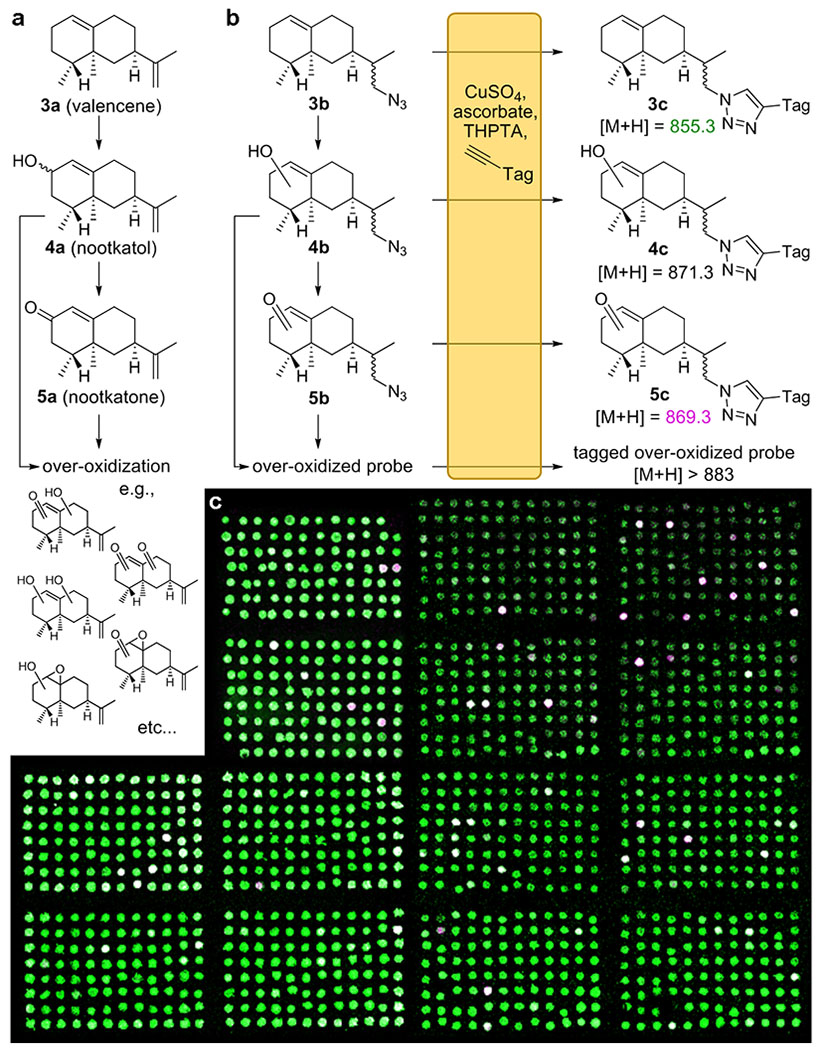
a) The P450-catalyzed oxidation of 3a to produce 4a, 5a, and other oxidized isoprenoids b) Analogously, the PECAN probe 3b, a surrogate for 3a, may be expected undergo P450-catalyzed oxidations to form 4b, 5b and similarly over-oxidized products. Upon tagging and MS analysis, ions corresponding to 3c and its various oxidized forms are observed. Structures 4b, 4c, 5b, and 5c represent a variety of isomers that cannot be distinguished by PECAN. “Tag” is identical to that shown in Fig. 2. c) MS image resulting from the high-throughput PECAN screening campaign for ValHN3-oxidizing mutants of P450BM3. The image shows ions with m/z = 855 (3c) in green, and m/z = 869 (5c) in violet. White pixels indicate both ions are present. Each of the pixels’ two color intensities maps linearly to their respective ion’s percentile intensity in the MS images. Composite image of 3 MS imaging experiments shown.
We performed combinatorial site-saturation mutagenesis (NNK codons) on two P450BM3 amino acid residues commonly mutagenized due to their proximity to the active site heme: F87 and A328[30,37]. While a subset of this library has been assayed for 3a oxidation before[36], no study has further explored its amino acid space, likely due to limited experimental throughput. A lack of library sequence bias was verified by Sanger sequencing 10 randomly-picked colonies. Like others, we observed color differences between the different wells of our P450BM3 library (Fig. S5), which has been attributed to the ability of some variants to oxidize indole to form the dyes indigo and indirubin[38]. Throughout our study, we found no clear correlation between colored wells and the enzyme’s ability to oxidize 3a.
Employing 3b as our PECAN probe analog of 3a, we screened 1208 E. coli cell lysates generated from our P450BM3 library for the ability to oxidize 3b into products with the mass of 5b, in a 96-well format. Hits could be visually identified from the resulting MS image (Fig. 3c). For a quantitative analysis, we used the OpenMSI Arrayed Analysis Toolkit[39] to identify wells displaying m/z = 869 (i.e., 5c) relative ion intensities more than 10 standard deviations above eight GFP controls included on the same 96-well plate. These hits were de-replicated by Sanger sequencing to yield the variants listed in Table 1. The identified amino acid substitutions consist mostly of conservative small and hydrophobic residues. Several variants were recovered more than once. All variants were found to have 3b-oxidation activity in a confirmatory PECAN experiment (Table S1).
Table 1.
Variants of P450BM3 discovered in the PECAN screen, how many times each variant was discovered in the screen, and their ability to oxidize 3a in vitro, as determined by GC-MS.
| P450BM3 variant | Number of hits | % 4a[a,b] | % 5a[a] | % Mis-oxidized[a,c] | % Over-oxidized[a,d] |
|---|---|---|---|---|---|
| Wild-type | 0 | 0.5 ±0.1 | ND[e] | 0.15 ±0.01 | ND |
| F87A/A238I[f] | 2 | 7.5 ±0.6 | 13.7 ±0.2 | 0.7 ±0.2 | 1.9 ±0.2 |
| F87G/A238V | 6 | 9.1 ±0.7 | 11.2 ±0.7 | 0.34 ±0.03 | 6.7 ±0.1 |
| F87P | 2 | 7.6 ±0.2 | 7.3 ±0.1 | 11.4 ±1.9 | 59.9 ±2.8 |
| F87A | 1 | 7.2 ±0.7 | 5.8 ±0.7 | 14.9 ±2.3 | 48.8 ±3.2 |
| F87I | 1 | 7.5 ±0.2 | 4.8 ±0.1 | 14.8 ±1.6 | 43.1 ±5.9 |
| F87G/A238L | 2 | 7.9 ±1.3 | 4.2 ±0.6 | 1.5 ±0.1 | 0.6 ±0.1 |
| F87A/A238V[f] | 1 | 7.2 ±0.4 | 4.0 ±0.2 | 0.41 ±0.05 | ND |
| F87G/A238S | 1 | 5.1 ±0.5 | 3.8 ±0.3 | 2.0 ±0.1 | 10.8 ±1.1 |
| F87G/A238N | 2 | 4.3 ±0.8 | 3.3 ±0.5 | 1.2 ±0.2 | 2.2 ±0.2 |
| F87V | 1 | 9.6 ±0.6 | 1.6 ±0.1 | 5.6 ±0.5 | 3.9 ±0.6 |
| F87T | 3 | 2.3 ±0.2 | 1.0 ±0.1 | 0.48 ±0.05 | 0.26 ±0.08 |
| F87G | 1 | 3.8 ±0.1 | 0.95 ±0.07 | 8.9 ±0.4 | 32.0 ±0.3 |
| F87A/A238Y | 1 | 4.1 ±0.9 | 0.38 ±0.07 | 0.03 ±0.05 | ND |
| F87I/A238L | 1 | 3.5 ±0.6 | 0.21 ±0.03 | 0.4 ±0.2 | 0.07 ±0.06 |
| F87G/A238P | 1 | 1.4 ±0.6 | 0.13 ±0.07 | 0.12 ±0.02 | ND |
| F87G/A238T | 6 | 1.1 ±0.3 | 0.11 ±0.04 | 0.10 ±0.02 | ND |
| F87G/A238G | 3 | ND | ND | 8.4 ±1.9 | 64.4 ±13.0 |
| F87A/A238P | 2 | 1.5 ±0.2 | ND | ND | ND |
| F87V/A238P | 2 | ND | ND | ND | ND |
In vitro oxidation of 0.5 mM 3a by 1 µM purified P450BM3 variant (i.e., 0.2 mol% catalyst loading) and a cofactor generation system capable of producing 10 mM NADPH, in 1 h, as percent of total GC-MS peak area. Unreacted 3a makes up the remainder. See Table S1 for a further breakdown
Sum of diastereomers.
Sum of all other oxidation products with the same nominal masses as 4a (220 Da) or 5a (218 Da) (See Fig. S4).
Sum of all oxidation products with masses higher than 220 Da, which may include products not derived from 4a or 5a.
Not Detected.
Because the PECAN screen could not distinguish between isomers, and because 4a is merely a surrogate substrate, we used GC-MS to assess the ability of P450BM3 variant hits to oxidize 3a in vitro (Table 1). Purified enzymes were employed to control for differences in protein expression levels. Nearly all screen hits were able to catalyze the conversion of 3a to 5a to some extent, whereas wild-type P450BM3 cannot. P450MB3 F87A, F87G, F87I, F87P and F87G/A238G were found to be hyper-active non-specific 3a oxidizers, which is unsurprising considering the small amino acid residues lining these variants’ active sites. Three variants did not produce 5a, of which only one variant (F87V/A238P) was completely inactive on 3a.
Two of our hits, F87A/A283I and F87A/A283V have previously been identified by Seifert et al, who constructed an “enriched” library of P450BM3 mutants substituting positions F87 and A328 with A, I, L, V and F combinatorially (25 variants total) and tested for the ability to convert 3a to 5a using GC-MS. This verifies that the PECAN technology can identify enzymes with desired catalytic activities that could previously be discovered only using low-throughput approaches. However, despite our ability to screen a significantly more comprehensive mutant library using the PECAN technology, we still identified F87A/A328I as the optimal nootkatone-producing P450BM3 variant, suggesting that the “enriched library” strategy employed by Seifert et al. was indeed effective. Other amino acid substitutions yielding variants with substantial nootkatone production are S, T, Y, N and G. Glycine, in particular, appears in many highly-active variants, suggesting that future “enriched” site-saturation enzyme libraries may benefit from including this amino acid.
While the PECAN screen was able to identify P450BM3 variants capable of producing nootkatone, some hits were false positives, and the relative intensities of oxidation products observed using the PECAN screen (e.g., 4c vs 5c) did not always match those measured by GC-MS (e.g., 4a vs 5a, Table S1). This is likely because probe 3b is not a perfect surrogate for 3a. Compared to 3a, which has 15 heavy atoms, 3b has 18, presenting a 20% increase in size. Larger enzyme substrates, when similarly functionalized with a “clickable” functional group, would be expected to yield probes more representative of their archetype. To further improve hit identification, PECAN could be used in conjunction with P450 fingerprinting, in which enzyme variants’ activities on multiple probes are correlated to activities on the substrate of interest measured for a subset of the library[40–42]. Alternatively, “label-free” technologies enabling the tagging of unfunctionalized biomolecules[43,44] promise to avoid this bias altogether.
While the potential for high-throughput NIMS-based analysis of complex enzyme reaction mixtures has frequently been alluded to, thus far all experiments describing the analysis of complex samples using NIMS have been limited to small studies[8,9,12], and all successful high-throughput NIMS experiments have been conducted with purified protein in low ionic strength buffer, avoiding the need for in situ fluorous affinity purification[43,45,46]. The screening campaign described here is the first demonstration of a high-throughput NIMS-based analysis of enzyme activity in a complex matrix.
In short, we have introduced the PECAN mass-spectrometric enzyme activity assay, and demonstrated its suitability for high-throughput screening of P450s in complex matrices such as cell lysates and microsomes. While here we have demonstrated the applicability of PECAN only to P450s, we believe that, after the appropriate method development, this technology could be applicable to any enzyme (or set of enzymatic reactions), that changes the mass of its substrate. Additionally, it may be possible to monitor isomerization reactions through the application of tandem MS, or enantioselective reactions through the use of isotopically labelled substrates[47]. As expected from any high-throughput assay, our PECAN screen yielded some false positives, and we re-screened a small subset of the library using low-throughput analytical technologies to verify the hits, as is routine in this field. Like spectrometric assays, the PECAN technology requires the synthesis of a specialized probe and enzymatic reactions are performed in microtiter plates. Therefore, we expect PECAN to have roughly equivalent throughput to – and act as a complementary method to – spectrometric assays. We expect PECAN to be a valuable technology for drug discovery or directed evolution campaigns that focus on enzymes for which no high-throughput screen currently exists.
Supplementary Material
Acknowledgements
This work was funded by National Institutes of Health Awards 1RC1GM090980, F32GM125179 and F32GM129960, National Science Foundation grants 1341894 and 1442724, and was part of the U.S. Department of Energy (DOE) Joint BioEnergy Institute (http://www.jbei.org) supported by the U.S. DOE, Office of Science, Office of Biological and Environmental Research, through contract DE-AC02-05CH11231 between Lawrence Berkeley National Laboratory and the U.S. Department of Energy. We would like to thank Daniel Liu, Joel Guenther, Pamela Peralta-Yahya, Benjamin Bowen, Carolyn Bertozzi, and Judith Klinman for insightful discussions.
Footnotes
Supporting information for this article is given via a link at the end of the document
Contributor Information
Tristan de Rond, College of Chemistry, University of California, Berkeley, Berkeley, CA 94270 (USA); Joint Bioenergy Institute (JBEI), Lawrence Berkeley National Laboratory, Emeryville, CA 94608 (USA); Current Affiliation: Scripps Institution of Oceanography, University of California, San Diego, La Jolla, CA 92037 (USA).
Jian Gao, Department of Energy Joint Genome Institute (DOE JGI), Lawrence Berkeley National Laboratory.
Amin Zargar, Joint Bioenergy Institute (JBEI), Lawrence Berkeley National Laboratory, Emeryville, CA 94608 (USA).
Markus de Raad, Department of Energy Joint Genome Institute (DOE JGI), Lawrence Berkeley National Laboratory; Environmental Genomics and Systems Biology, Lawrence Berkeley National Laboratory.
Jack Cunha, Joint Bioenergy Institute (JBEI), Lawrence Berkeley National Laboratory, Emeryville, CA 94608 (USA).
Trent R. Northen, Joint Bioenergy Institute (JBEI), Lawrence Berkeley National Laboratory, Emeryville, CA 94608 (USA); Department of Energy Joint Genome Institute (DOE JGI), Lawrence Berkeley National Laboratory; Environmental Genomics and Systems Biology, Lawrence Berkeley National Laboratory
Jay D. Keasling, College of Chemistry, University of California, Berkeley, Berkeley, CA 94270 (USA); Joint Bioenergy Institute (JBEI), Lawrence Berkeley National Laboratory, Emeryville, CA 94608 (USA); Biological Systems and Engineering Division, Lawrence Berkeley National Laboratory; Center for Biosustainability, Danish Technical University, Lyngby, Denmark; Center for Synthetic Biochemistry, Institute for Synthetic Biology, Shenzhen Institutes of Advanced Technology, Shenzhen, China.
References
- [1].Reetz MT, in Directed Evolution of Selective Enzymes: Catalysts for Organic Chemistry and Biotechnology, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2016, pp. 27–57. [Google Scholar]
- [2].Jacques P, Béchet M, Bigan M, Caly D, Chataigné G, Coutte F, Flahaut C, Heuson E, Leclère V, Lecouturier D, et al. , Bioprocess Biosyst Eng 2017, 40, 161–180. [DOI] [PubMed] [Google Scholar]
- [3].Macarron R, Banks MN, Bojanic D, Burns DJ, Cirovic DA, Garyantes T, Green DVS, Hertzberg RP, Janzen WP, Paslay JW, et al. , Nat. Rev. Drug Discov. 2011, 10, 188–195. [DOI] [PubMed] [Google Scholar]
- [4].Turner NJ, Nat. Chem. Biol. 2009, 5, 567–573. [DOI] [PubMed] [Google Scholar]
- [5].Sun H, Zhang H, Ang EL, Zhao H, Bioorg. Med. Chem. 2018, 26, 1275–1284. [DOI] [PubMed] [Google Scholar]
- [6].Packer MS, Liu DR, Nat. Rev. Genet. 2015, 16, 379–394. [DOI] [PubMed] [Google Scholar]
- [7].Dietrich JA, McKee AE, Keasling JD, Annu. Rev. Biochem. 2010, 79, 563–590. [DOI] [PubMed] [Google Scholar]
- [8].Northen TR, Lee J-C, Hoang L, Raymond J, Hwang D-R, Yannone SM, Wong C-H, Siuzdak G, Proc. Natl. Acad. Sci. USA 2008, 105, 3678–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].de Rond T, Peralta-Yahya P, Cheng X, Northen TR, Keasling JD, Anal. Bioanal. Chem. 2013, 405, 4969–4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Northen TR, Yanes O, Northen MT, Marrinucci D, Uritboonthai W, Apon J, Golledge SL, Nordström A, Siuzdak G, Nature 2007, 449, 1033–1036. [DOI] [PubMed] [Google Scholar]
- [11].Deng K, George KW, Reindl W, Keasling JD, Adams PD, Lee TS, Singh AK, Northen TR, Rapid Commun. Mass Spectrom. 2012, 26, 611–615. [DOI] [PubMed] [Google Scholar]
- [12].Reindl W, Deng K, Gladden JM, Cheng G, Wong A, Singer SW, Singh S, Lee JC, Yao CH, Hazen TC, et al. , Energy Environ. Sci. 2011, 4, 2884. [Google Scholar]
- [13].de Rond T, Danielewicz M, Northen T, Curr. Opin. Biotechnol. 2015, 31, 1–9. [DOI] [PubMed] [Google Scholar]
- [14].Rostovtsev VV, Green LG, Fokin VV, Sharpless KB, Angew. Chem. Int. Ed. 2002, 41, 2596–2599; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2002, 114, 2708–2711 [Google Scholar]
- [15].Greving M, Cheng X, Reindl W, Bowen B, Deng K, Louie K, Nyman M, Cohen J, Singh A, Simmons B, et al. , Anal. Bioanal. Chem. 2012, 403, 707–711. [DOI] [PubMed] [Google Scholar]
- [16].Podust LM, Sherman DH, Nat Prod Rep 2012, 29, 1251–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Niwa T, Murayama N, Imagawa Y, Yamazaki H, Drug Metab Rev 2015, 47, 89–110. [DOI] [PubMed] [Google Scholar]
- [18].De Montellano PRO, Cytochrome P450: Structure, Mechanism, and Biochemistry, Springer Science & Business Media, 2005. [Google Scholar]
- [19].Girvan HM, Munro AW, Curr. Opin. Chem. Biol. 2016, 31, 136–145. [DOI] [PubMed] [Google Scholar]
- [20].Urlacher VB, Girhard M, Trends Biotechnol. 2012, 30, 26–36. [DOI] [PubMed] [Google Scholar]
- [21].Fasan R, ACS Catal. 2012, 2, 647–666. [Google Scholar]
- [22].Farinas E, Schwaneberg U, Glieder A, Arnold F, Adv Synth Catal 2001, 343, 601–606. [Google Scholar]
- [23].Peters MW, Meinhold P, Glieder A, Arnold FH, J. Am. Chem. Soc. 2003, 125, 13442–13450. [DOI] [PubMed] [Google Scholar]
- [24].Fasan R, Chen MM, Crook NC, Arnold FH, Angew. Chem. Int. Ed. 2007, 46, 8414–8418; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2007, 119, 8566–8570 [Google Scholar]
- [25].Crespi CL, Miller VP, Stresser DM, in Cytochrome P450 Part C, Elsevier, 2002, pp. 276–284. [DOI] [PubMed] [Google Scholar]
- [26].Cali JJ, Ma D, Sobol M, Simpson DJ, Frackman S, Good TD, Daily WJ, Liu D, Expert Opin Drug Metab Toxicol 2006, 2, 629–645. [DOI] [PubMed] [Google Scholar]
- [27].Tang WL, Li Z, Zhao H, Chem. Commun. 2010, 46, 5461–5463. [DOI] [PubMed] [Google Scholar]
- [28].Furuya T, Nishi T, Shibata D, Suzuki H, Ohta D, Kino K, Chem. Biol. 2008, 15, 563–572. [DOI] [PubMed] [Google Scholar]
- [29].Lim KB, Ozbal CC, Kassel DB, Methods Mol. Biol. 2013, 987, 25–50. [DOI] [PubMed] [Google Scholar]
- [30].Whitehouse CJC, Bell SG, Wong L-L, Chem. Soc. Rev. 2012, 41, 1218–1260. [DOI] [PubMed] [Google Scholar]
- [31].Agudo R, Roiban G-D, Reetz MT, Chembiochem 2012, 13, 1465–1473. [DOI] [PubMed] [Google Scholar]
- [32].Zhang JH, Chung TD, Oldenburg KR, J. Biomol. Screen. 1999, 4, 67–73. [DOI] [PubMed] [Google Scholar]
- [33].Zhu BC, Henderson G, Chen F, Maistrello L, Laine RA, J. Chem. Ecol. 2001, 27, 523–531. [DOI] [PubMed] [Google Scholar]
- [34].Fraatz MA, Berger RG, Zorn H, Appl. Microbiol. Biotechnol. 2009, 83, 35–41. [DOI] [PubMed] [Google Scholar]
- [35].Sowden RJ, Yasmin S, Rees NH, Bell SG, Wong L-L, Org. Biomol. Chem. 2005, 3, 57–64. [DOI] [PubMed] [Google Scholar]
- [36].Seifert A, Vomund S, Grohmann K, Kriening S, Urlacher VB, Laschat S, Pleiss J, Chembiochem 2009, 10, 853–861. [DOI] [PubMed] [Google Scholar]
- [37].Seifert A, Pleiss J, Proteins 2009, 74, 1028–1035. [DOI] [PubMed] [Google Scholar]
- [38].Li QS, Schwaneberg U, Fischer P, Schmid RD, Chem. Eur. J 2000, 6, 1531–1536. [DOI] [PubMed] [Google Scholar]
- [39].de Raad M, de Rond T, Rübel O, Keasling JD, Northen TR, Bowen BP, Anal. Chem. 2017, 89, 5818–5823. [DOI] [PubMed] [Google Scholar]
- [40].Zhang K, El Damaty S, Fasan R, J. Am. Chem. Soc. 2011, 133, 3242–3245. [DOI] [PubMed] [Google Scholar]
- [41].Zhang K, Shafer BM, Demars MD, Stern HA, Fasan R, J. Am. Chem. Soc. 2012, 134, 18695–18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kolev JN, O’Dwyer KM, Jordan CT, Fasan R, ACS Chem. Biol. 2014, 9, 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Deng K, Takasuka TE, Heins R, Cheng X, Bergeman LF, Shi J, Aschenbrener R, Deutsch S, Singh S, Sale KL, et al. , ACS Chem. Biol. 2014, 9, 1470–1479. [DOI] [PubMed] [Google Scholar]
- [44].Helal KY, Alamgir A, Berns EJ, Mrksich M, J. Am. Chem. Soc. 2018, 140, 8060–8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Heins RA, Cheng X, Nath S, Deng K, Bowen BP, Chivian DC, Datta S, Friedland GD, D’Haeseleer P, Wu D, et al. , ACS Chem. Biol. 2014, 9, 2082–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Deng K, Guenther JM, Gao J, Bowen BP, Tran H, Reyes-Ortiz V, Cheng X, Sathitsuksanoh N, Heins R, Takasuka TE, et al. , Front. Bioeng. Biotechnol. 2015, 3, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Chen Y, Tang WL, Mou J, Li Z, Angew. Chem. Int. Ed. 2010, 49, 5278–5283; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2010, 122, 5406–5411 [Google Scholar]
- [48].Seifert A, Vomund S, Grohmann K, Kriening S, Urlacher VB, Laschat S, Pleiss J, Chembiochem 2009, 10, 1426–1426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


