Table 1.
Optimization of reaction conditions.[a]
 | ||||
|---|---|---|---|---|
| entry | x (eq.) | [Pd] | ligand | yield (%)[b] |
| 1 | 2.5 | Pd(PPh3)4 | – | 35 |
| 2 | 2.5 | Pd(dba)2 | dppf | <5 |
| 3 | 2.5 | Pd(dba)2 | P(p-FC6H4)3 | 34 |
| 4 | 2.5 | Pd(dba)2 | P(p-MeC6H4)3 | <5 |
| 5 | 2.5 | Pd(dba)2 | P(o-MeC6H4)3 | 63 |
| 6 | 1.5 | Pd(dba)2 | P(o-MeC6H4)3 | 63 |
| 7[c] | 1.5 | Pd(dba)2 | P(o-MeC6H4)3 | 61 |
| 8[c,d] | 1.5 | Pd(dba)2 | P(o-MeC6H4)3 | 78 (75) |
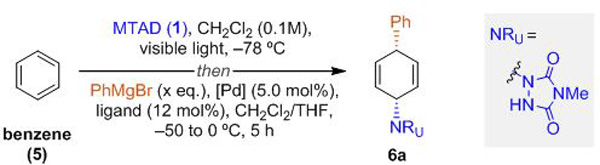
Reaction conditions: benzene (5, 5.0 mmol, 10 eq.), MTAD (1, 0.5 mmol, 1.0 eq.), CH2Cl2 (0.1 M), visible light, –78 ºC; then addition of PhMgBr (3.0 M in Et2O), [Pd] catalyst in THF.
Determined by 1H NMR analysis relative to the internal standard. Isolated yield shown in parentheses.
CH2Cl2 (0.2 M).
Substitution step was conducted from –50 to 15 ºC over 10 h.
