FIGURE 3.
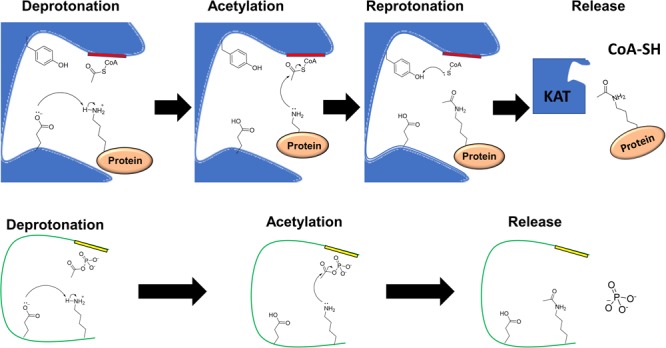
Catalytic mechanisms of enzymatic and non-enzymatic acetylation. (Top) In the enzymatic mechanism, a lysine to be acetylated binds at the acceptor site of a lysine acetyltransferase (KAT) and AcCoA binds at the donor site of the KAT called the P-loop (red) with consensus motif Gln/Arg-x-x-Gly-x-Gly/Ala (Salah Ud-Din et al., 2016). A catalytic glutamate deprotonates the epsilon amino group of the target lysine. The lysine performs a nucleophilic attack on the carbonyl carbon of AcCoA, resulting in acetylation of the lysine. The CoA group becomes protonated by a tyrosine, which regenerates the KAT and facilitates the release of the free CoA and the target protein. (Bottom) In this example of non-enzymatic acetylation, AcP is bound through its negatively charged phosphoryl group to a neighboring patch (yellow) that contains positively charged residues and/or residues that can form hydrogen bonds. In this example, the lysine is deprotonated by a glutamate on the same protein. The lysine performs a nucleophilic attack on the carbonyl carbon of AcP resulting in an acetyllysine. Inorganic phosphate is released as a byproduct of the reaction. A mechanism similar to this can be considered for non-enzymatic AcCoA-dependent acetylation.
