Figure 3.
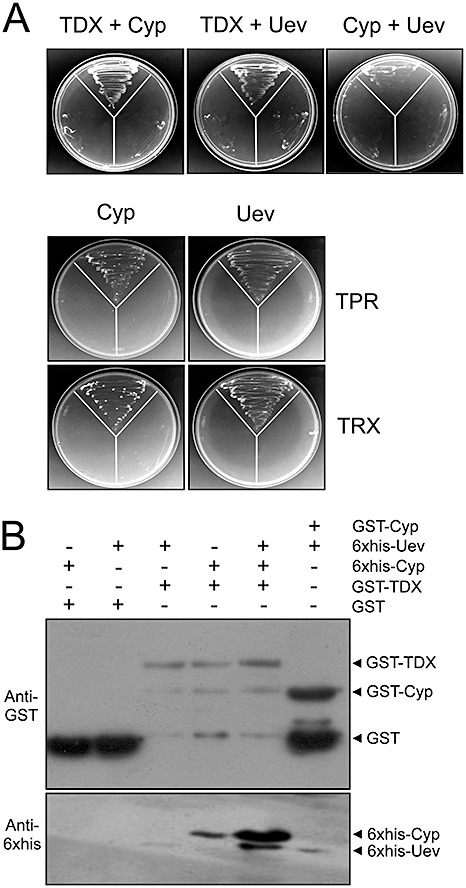
The citrus proteins cyclophilin (Cyp), thioredoxin (TDX) and ubiquitin‐conjugating enzyme variant (Uev) interact with each other. (A) Yeast two‐hybrid assays showing interactions between TDX, Cyp and Uev, as well as between Cyp and Uev. The tetratricopeptide repeat (TPR) and thioredoxin (TRX) domains of TDX also interact with Cyp and Uev. The disposition of the yeast co‐transformants in the plates is the same as shown in Fig. 1. (B) Western blot of glutathione transferase (GST) pulldown assays using GST–TDX or GST–Cyp as bait and 6 × His–Cyp and 6 × His–Uev as prey. Consistent with the in vivo assay shown in (A), stronger interactions between TDX and Cyp are observed in the presence of Uev, suggesting the existence of a protein complex formed by TDX, Cyp and Uev. The corresponding sodium dodecylsulphate (SDS) gel is shown in Fig. S2 (see Supporting Information).
